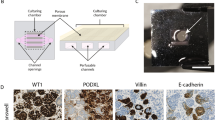
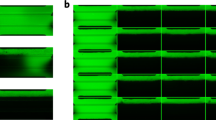
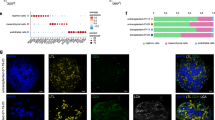
Abstract
Kidney organoids derived from human pluripotent stem cells have glomerular- and tubular-like compartments that are largely avascular and immature in static culture. Here we report an in vitro method for culturing kidney organoids under flow on millifluidic chips, which expands their endogenous pool of endothelial progenitor cells and generates vascular networks with perfusable lumens surrounded by mural cells. We found that vascularized kidney organoids cultured under flow had more mature podocyte and tubular compartments with enhanced cellular polarity and adult gene expression compared with that in static controls. Glomerular vascular development progressed through intermediate stages akin to those involved in the embryonic mammalian kidney’s formation of capillary loops abutting foot processes. The association of vessels with these compartments was reduced after disruption of the endogenous VEGF gradient. The ability to induce substantial vascularization and morphological maturation of kidney organoids in vitro under flow opens new avenues for studies of kidney development, disease, and regeneration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data generated in this study are available from the corresponding authors upon request.
References
Morizane, R. & Bonventre, J. V. Generation of nephron progenitor cells and kidney organoids from human pluripotent stem cells. Nat. Protoc. 12, 195–207 (2017).
Morizane, R. et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 33, 1193–1200 (2015).
Takasato, M. et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568 (2015).
Lam, A. Q. et al. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J. Am. Soc. Nephrol. 25, 1211–1225 (2014).
Freedman, B. S. et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat. Commun. 6, 8715 (2015).
Morizane, R. & Bonventre, J. V. Kidney organoids: a translational journey. Trends Mol. Med. 23, 246–263 (2017).
Taguchi, A. et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53–67 (2014).
Takasato, M. & Little, M. H. A strategy for generating kidney organoids: recapitulating the development in human pluripotent stem cells. Dev. Biol. 420, 210–220 (2016).
Wu, H. et al. Comparative analysis of kidney organoid and adult human kidney single cell and single nucleus transcriptomes. bioRxiv Preprint at https://www.biorxiv.org/content/early/2017/12/11/232561 (2017).
van den Berg, C. W. et al. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Rep. 10, 751–765 (2018).
Takebe, T. et al. Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell 16, 556–565 (2015).
Bantounas, I. et al. Generation of functioning nephrons by implanting human pluripotent stem cell-derived kidney progenitors. Stem Cell Rep. 10, 766–779 (2018).
Camp, J. G. et al. Multilineage communication regulates human liver bud development from pluripotency. Nature 546, 533–538 (2017).
Kolesky, D. B. et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 26, 3124–3130 (2014).
Homan, K. A. et al. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci. Rep. 6, 34845 (2016).
Halt, K. J. et al. CD146+ cells are essential for kidney vasculature development. Kidney Int. 90, 311–324 (2016).
Zudaire, E., Gambardella, L., Kurcz, C. & Vermeren, S. A computational tool for quantitative analysis of vascular networks. PLoS One 6, e27385 (2011).
Munro, D. A. D., Hohenstein, P. & Davies, J. A. Cycles of vascular plexus formation within the nephrogenic zone of the developing mouse kidney. Sci. Rep. 7, 3273 (2017).
Daniel, E. et al. Spatiotemporal heterogeneity and patterning of developing renal blood vessels. Angiogenesis 21, 617–634 (2018).
Robert, B., St John, P. L. & Abrahamson, D. R. Direct visualization of renal vascular morphogenesis in Flk1 heterozygous mutant mice. Am. J. Physiol. 275, F164–F172 (1998).
McMahon, A. P. Development of the mammalian kidney. Curr. Top. Dev. Biol. 117, 31–64 (2016).
Abrahamson, D. R. Development of kidney glomerular endothelial cells and their role in basement membrane assembly. Organogenesis 5, 275–287 (2009).
Scheppke, L. et al. Notch promotes vascular maturation by inducing integrin-mediated smooth muscle cell adhesion to the endothelial basement membrane. Blood 119, 2149–2158 (2012).
Wu, S., Kim, C., Baer, L. & Zhu, X. Bevacizumab increases risk for severe proteinuria in cancer patients. J. Am. Soc. Nephrol. 21, 1381–1389 (2010).
Sharmin, S. et al. Human induced pluripotent stem cell-derived podocytes mature into vascularized glomeruli upon experimental transplantation. J. Am. Soc. Nephrol. 27, 1778–1791 (2016).
Serluca, F. C., Drummond, I. A. & Fishman, M. C. Endothelial signaling in kidney morphogenesis: a role for hemodynamic forces. Curr. Biol. 12, 492–497 (2002).
Ichimura, K. et al. Morphological process of podocyte development revealed by block-face scanning electron microscopy. J. Cell. Sci. 130, 132–142 (2017).
Huch, M., Knoblich, J. A., Lutolf, M. P. & Martinez-Arias, A. The hope and the hype of organoid research. Development 144, 938–941 (2017).
Oxburgh, L. & Carroll, T. J. The bioengineered kidney: science or science fiction? Curr. Opin. Nephrol. Hypertens. 25, 343–347 (2016).
Little, M. H. Growing kidney tissue from stem cells: how far from “party trick” to medical application? Cell Stem Cell 18, 695–698 (2016).
Ainslie, K. M., Garanich, J. S., Dull, R. O. & Tarbell, J. M. Vascular smooth muscle cell glycocalyx influences shear stress-mediated contractile response. J. Appl. Physiol. 98, 242–249 (2005).
Acknowledgements
The authors thank P. Galichon for flow cytometry analyses; Y. Yoda and K. Susa for cell culture and immunocytochemistry; S. Jain at The Washington University Kidney Translational Research Center (KTRC; St. Louis, MO, USA) for providing the BJFF hiPSC line; A. Moisan, C. Chen, and S. Uzel for insightful discussions; J. Weaver, B. Roman-Manso, N. Zhou, and M. Ericsson for imaging assistance; and L. Sanders for videography. This study was supported by the US National Institutes of Health (NIH; T32 fellowship training grant DK007527 to N.G.; Subaward U01DK107350 to M.T.V.; R37 grant DK039773 to J.V.B.; UG3 grant TR002155 to J.V.B., M.T.V., J.A.L., and R.M.; grant P30 DK079333 (the BJFF line) supporting The Washington University KTRC), the Harvard Stem Cell Institute (interdisciplinary grant to N.G.; seed grant to R.M. and J.A.L.), Brigham and Women’s Hospital (Research Excellence Award to N.G. and R.M.; Faculty Career Development Award to R.M.), the NIDDK Diabetic Complications Consortium (DiaComp, https://www.diacomp.org; grant DK076169 to R.M.), the NIH (Re)Building a Kidney Consortium (U01DK107350 to K.A.H. and J.A.L.), the Office of Naval Research Vannevar Bush Faculty Fellowship program (award no. N000141612823 to M.S.-S. and J.A.L.), and the Wyss Institute for Biologically Inspired Engineering (D.B.K., K.T.K., D.M., and J.A.L.). J.A.L. thanks the GETTYLAB and S. Lindenfeld for their generous donations in support of this research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
K.A.H., N.G., J.A.L., R.M., D.B.K., and J.V.B. conceived the project. K.A.H., N.G., and K.T.K. designed the research, and R.M. and J.A.L. supervised the research. K.A.H., N.G., K.T.K., R.M., and D.B.K. designed, performed, and analyzed all experiments. M.T.V. provided critical insights into embryonic development, cell sources, and mouse embryonic kidneys. M.S.-S. designed and built the silicone millifluidic chips, interfacing with perfusion pumps, and analyzed the fluid flow profiles on chip. D.M. and T.M. sourced and validated antibodies, optimized staining protocols, and provided invaluable cell culture analysis and support. T.F. developed methodology for quantifying vascular and tubule features in confocal imaging stacks. All authors contributed to manuscript writing.
Corresponding authors
Ethics declarations
Competing interests
J.V.B. and R.M. are co-inventors on patents (PCT/US16/52350) on organoid technologies that are assigned to Partners Healthcare. J.V.B. or his family has received income for consulting from companies interested in biomarkers: Sekisui, Millennium, Johnson & Johnson, and Novartis. J.V.B. is a co-founder of, is a consultant to, and owns equity in Goldfinch Bio. K.A.H. is a co-founder and chairwoman of NanoHybrids Inc. J.A.L. is a co-founder of and owns equity in Voxel8 Inc.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–22 and Supplementary Tables 1–3
Supplementary Video 1
Vascular precursors and mature vessels in a kidney organoid cultured under high -flow conditions. Confocal z-stack movie of a whole organoid, containing multiple PODXL+ cellular clusters, permeated by PECAM1+ vessels supported by MCAM+ cells. DAPI (blue), 4’,6-diamidino-2-phenylindole; PECAM1 (red), CD31; MCAM (yellow), CD146; PODXL (cyan), podocalyxin.
Supplementary Video 2
Bead perfusion at the bottom edge of a live organoid. Beads (green; 100 nm) were perfused in media over the top of an organoid in high-flow conditions. The beads accumulate with time at the edge (white dotted line). ULEX staining (red) was applied to the media and highlights the vessels. Halfway through the movie, the red channel laser is turned off in order to visualize the beads in the green channel only. As is common with this system, one vessel is perfused and a nearby vessel is not, showing that perfusion in lumens in this system is happening, but not evenly throughout the vascular tree. Scale bar, 10 μm.
Supplementary Video 3
Kidney organoid fusion under flow. Bright-field time-lapse movie of two organoids fusing after being seeded on chip under high flow during a 47-h period (images taken every 2 min). (Note: the period shown corresponds to days 1–3 under perfusion.)
Supplementary Video 4
Vascular–tubule interactions in kidney organoids under high flow. PECAM1+ cells (red) both wrap and extend longitudinally around tubules. DAPI (blue), 4’,6-diamidino-2-phenylindole; PECAM1 (red), CD31; PODXL (cyan), podocalyxin.
Supplementary Video 5
Capillary invasion into S-shaped bodies and PODXL+ lobules in kidney organoids cultured under high flow. Z-stack confocal slices through various glomeruli in organoids under high-flow conditions (z-stacks of the still images in Fig. 4d,e in the text).
Supplementary Video 6
Confocal z-stack movies of PODXL+ lobules with PECAM1+ vessels supported by MCAM+ cells for kidney organoids cultured under high flow at day 21. DAPI (blue), 4’,6-diamidino-2-phenylindole; PECAM1 (red), CD31; MCAM (yellow), CD146; PODXL (cyan), podocalyxin.
Rights and permissions
About this article
Cite this article
Homan, K.A., Gupta, N., Kroll, K.T. et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods 16, 255–262 (2019). https://doi.org/10.1038/s41592-019-0325-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-019-0325-y
This article is cited by
-
Utilizing bioprinting to engineer spatially organized tissues from the bottom-up
Stem Cell Research & Therapy (2024)
-
Microfluidic high-throughput 3D cell culture
Nature Reviews Bioengineering (2024)
-
Neuropathogenesis-on-chips for neurodegenerative diseases
Nature Communications (2024)
-
Generating human bone marrow organoids for disease modeling and drug discovery
Nature Protocols (2024)
-
Role of biophysics and mechanobiology in podocyte physiology
Nature Reviews Nephrology (2024)