Abstract
There is a paucity of high-quality evidence on the effectiveness and safety of salt reduction strategies, particularly for older people, who have the most to benefit but are at higher risk of adverse effects. Here, we conducted a clinical trial in which 48 residential elderly care facilities in China (1,612 participants including 1,230 men and 382 women, 55 years or older) were cluster-randomized using a 2 × 2 factorial design to provision of salt substitute (62.5% NaCl and 25% KCl) versus usual salt and to a progressively restricted versus usual supply of salt or salt substitute for 2 years. Salt substitute compared with usual salt lowered systolic blood pressure (–7.1 mmHg, 95% confidence interval (CI) –10.5 to –3.8), meeting the primary outcome of the trial, whereas restricted supply compared with usual supply of salt or salt substitute had no effect on systolic blood pressure. Salt substitute also lowered diastolic blood pressure (–1.9 mmHg, 95% CI –3.6 to –0.2) and resulted in fewer cardiovascular events (hazard ratio (HR) 0.60, 95% CI 0.38–0.96), but had no effect on total mortality (HR 0.84, 95% CI 0.63–1.13). From a safety standpoint, salt substitute increased mean serum potassium and led to more frequent biochemical hyperkalemia, but was not associated with adverse clinical outcomes. In contrast, salt restriction had no effect on any study outcome. The results of this trial indicate that use of salt substitute, but not efforts to restrict salt supply, may achieve blood pressure lowering and deliver health benefits to residents of elderly care facilities in China. Clinicaltrials.gov registration: NCT03290716
Similar content being viewed by others
Main
High blood pressure (BP) is a leading cause of death1, and there is clear evidence that lowering dietary sodium intake and increasing dietary potassium intake can reduce BP2,3. Sodium consumption in China is high4 and salt substitution is a proven nonpharmaceutical intervention for BP reduction in China5. Studies of salt substitute among older populations, who are at the greatest risk and have most to benefit, are few6. In addition, there have been concerns about the risk of hyperkalemia7,8,9, but safety data are limited10. Modeling studies projecting effects of salt substitution in China have indicated great potential benefits for cardiovascular disease (CVD) and death11,12, but data from large trials have been lacking until recently13.
Progressive reduction in the use of salt for the preparation and seasoning of food is a recommended strategy for reducing dietary sodium consumption14. Small, stepwise declines in the sodium content of foods could cumulate to large decreases, while not being perceived by consumers15. In addition, sustained reduction of salt consumption may result in adaptive change in taste preference for a lower-salt diet. Some small, short-term trials demonstrated that a one-quarter decrease in the sodium content of bread can be delivered unnoticed with gradual reduction16. However, strong evidence for the effectiveness and feasibility of this strategy from large-scale, long-term studies remains lacking.
The Diet, ExerCIse and carDiovascular hEalth (DECIDE)–Salt Reduction Strategies for Seniors in Residential Facilities (DECIDE-Salt) study aimed to use a factorial design to determine the effectiveness and safety of two practical and scalable sodium reduction intervention strategies in parallel, targeting older adults collectively living in residential elderly care facilities: (1) replacing usual salt with salt substitute and (2) making a stepwise reduction in the quantity of salt/salt substitute supplied to facility kitchens17.
Results
Study implementation and baseline characteristics
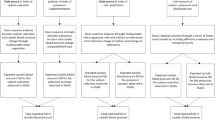
There were 48 facilities with a total of 1,612 eligible participants enrolled from 29 September 2017 to 28 March 2018 (Fig. 1). The participants had a mean age of 71.0 years and mean BP of 137.5 over 80.5 mmHg; 76.3% were men; 62.1% had a history of hypertension (HTN); 29.1% had a history of stroke or coronary artery disease; 19.4% had nonvascular health conditions; 26.2% were apparently healthy and 8.3% used medications that may elevate serum potassium. All baseline characteristics were balanced across the randomized groups (Table 1 and Extended Data Table 1).
Follow-up BP measurements at 6 (n = 903) and 18 (n = 799) months are not shown since follow-up visits were not completed in Xi’an at 6 and 18 months. aReasons for not participating in the baseline survey: 185 (49.2%) were temporarily absent from the facility, 33 (8.8%) were persons with disabilities, 59 (15.7%) were severely ill or bedridden, 32 (8.5%) refused the baseline survey and 67 (17.8%) had unknown reasons. bDrop out of the facility was due to administrative reasons (Methods). Reasons for missing follow-up BP measurements among a total of 2,841 missing measurements: no physical examination done at 6 and 18 months in Xi’an (29.0%), death (22.9%), permanent (17.0%) or temporary (15.0%) absence from the facility, refusal (5.1%) or unknown reasons (10.9%).
During follow-up, 249 participants died, 131 participants relocated out of the facilities, and two facilities with 54 participants (one from the group with no intervention and one from the group with both interventions) dropped out of the study. The deaths, checkouts and dropouts led to a substantial reduction in the number of eligible participants for physical and biochemical assessments at follow-up visits. In addition, in Xi’an the high proportions of participants who had severe illness or were bedridden, or lacked follow-up visits at 6 and 18 months also added to missing data. Among the 1,612 eligible participants, 1,219 (76%) had a measure for the primary BP outcome made on at least one follow-up visit but all 1,612 (100%) had clinical outcomes data available for analyses (Fig. 1). Participants missing follow-up BP measurements were on average older, more likely to be women, better educated, bedridden or severely ill at baseline, and were less likely to smoke, drink, have HTN or be using anti-HTN medication (Supplementary Table 2). These baseline characteristics did not differ between randomized comparisons in those with follow-up measures available (Supplementary Table 2).
Effects on primary efficacy outcome
Salt substitute compared with usual salt lowered mean systolic blood pressure (SBP), the primary outcome, by –7.1 mmHg (95% CI –10.5 to –3.8; Bonferroni corrected P < 0.001) (Extended Data Table 2 and Fig. 2). The estimates from the prespecified sensitivity analyses were –6.9 mmHg (95% CI –10.3 to –3.5) in the per-protocol analysis, –7.0 mmHg (95% CI –10.4 to –3.6) with exclusion of facilities from Xi’an and –6.6 mmHg (95% CI –10.3 to –2.8) with imputation for missing data (Extended Data Table 2). All results were statistically significant after adjustment for multiple comparisons using the Bonferroni method. The effects on SBP were greater for women than men and for the less educated compared with the more educated (both P homogeneity < 0.04). Effects also varied by tertiles of baseline SBP but with no clear pattern (P homogeneity = 0.05), and were not different between the other prespecified subgroups (all P homogeneity > 0.05; Extended Data Fig. 1).
a,b, Effects of salt substitute versus usual salt (a) and progressively restricted versus continued usual supply of salt or salt substitute (b) on SBP (top) and DBP (bottom) are shown. Data show mean and 95% CI values at baseline and each follow-up visit. The mean differences and 95% CI in SBP and DBP between comparison groups are based on BP measurements at four follow-up visits and were estimated using a linear mixed model with repeated measurements, accounting for clustering effects and adjusting for baseline values. The P value was two-sided and was not adjusted for multiple comparison. P values < 0.001 are reported as P < 0.001, instead of the actual exact P values.
There were no effects on the 2-year overall mean systolic blood pressure of restricted versus usual supply of salt/salt substitute in the primary analysis or any sensitivity analyses, though potential differences were noticed at 24 months (Fig. 2 and Extended Data Fig. 2).
Effects on secondary efficacy outcomes
The secondary efficacy outcomes were diastolic blood pressure (DBP), definite major cardiovascular events and total death. DBP was –1.9 mmHg (95% CI –3.6 to –0.2; P = 0.03) lower in those assigned to salt substitute compared with usual salt, with similar effect estimates in the sensitivity analyses (Fig. 2). There were no effects on DBP of restricted versus usual supply of salt/salt substitute.
There were 86 (5.4%) definite and 81 (5.0%) probable major cardiovascular events and 249 (15%) deaths recorded during the 2 years of the study. Definite cardiovascular events were reduced with salt substitute compared with usual salt (2.3 versus 3.8 per 100 person years (100pt-yrs), HR 0.60, 95% CI 0.38–0.96; P = 0.03) but no effect on total mortality was found (7.9 versus 9.4 per 100pt-yrs, HR 0.84, 95% CI 0.63–1.13; P = 0.24) (Fig. 3). Exploratory analyses based on all 167 definite or probable cardiovascular events (HR 0.66, 95% CI 0.48–0.90; P = 0.008) and 117 cardiovascular deaths (HR 0.64, 95% CI 0.44–0.92; P = 0.02) suggested benefits for those outcomes with salt substitute (Table 2). There were no effects on cardiovascular events or all-cause mortality for restricted versus usual supply of salt/salt substitute (all P values > 0.24) (Supplementary Table 5).
a,b, Effects of salt substitute versus usual salt (a) and progressively restricted versus continued usual supply of salt or salt substitute (b) on cardiovascular events (top) and total mortality (bottom). HR, 95% CI and P values were estimated from the Cox frailty model. The P value was two-sided and was not adjusted for multiple comparison.
Effects on safety outcomes
Safety was assessed among 1,086 participants with blood assays during follow-up. Participants without assays were more likely to be older, women, better educated, bedridden or severely ill (Supplementary Table 3) but there was no difference in baseline characteristics between randomized groups in those with and without blood assays.
Salt substitute compared with usual salt increased mean serum potassium by 0.26 mmol l–1 (95% CI 0.15–0.36; P < 0.001) and decreased mean serum sodium by –0.92 mmol l–1 (95% CI –1.43 to –0.41; P < 0.001). Compared with usual salt, the risk of biochemical hyperkalemia was increased with salt substitute (7.0% versus 2.4%, risk ratio (RR) 3.29, 95% CI 1.45–7.45; P = 0.004) with a corresponding fall in the risk of hypokalemia (0.7% versus 3.0%, RR 0.24, 95% CI 0.07–0.79; P = 0.02)—a supplementary safety outcome (Table 3). There was no different effect on the risk of biochemical hyperkalemia by age, sex, health status or hyperkalemia risk at baseline (all P values for interaction > 0.3) (Extended Data Fig. 3).
Among the 51 patients with biochemical hyperkalemia, two died—one from complications secondary to hip fracture in the intervention group and one from suspected lung cancer in the control group. In addition, among these 51 patients there were none in the intervention group and one in the control group who had a subsequent major cardiovascular event. Persistent biochemical hyperkalemia occurred in only three participants, none of whom died or experienced a cardiovascular event. No effects were observed on risk of incident hyponatremia (RR 1.60, 95% CI 0.48–5.41) or renal dysfunction (RR 1.41, 95% CI 0.47–4.24) (Table 3). There were no effects on safety outcomes with restricted supply (all P values > 0.05) (Supplementary Table 6).
Effects on process indicators
The prespecified process indicators were 24-h urinary sodium and potassium excretion, and 24-h urine samples were collected in a subset of participants at 24 months (639 participants). Participants with 24-h collections were systematically different from those who did not, but there were no differences in baseline characteristics between randomized groups in those with urine specimens (Supplementary Table 4).
Comparing participants receiving salt substitute versus usual salt, 24-h urinary potassium excretion was increased by 12.8 mmol (95% CI 5.7–19.8) but there was no detectable effect on 24-h urinary sodium excretion (–9.7 mmol; 95% CI –28.4 to 9.1). No differences on these urinary outcomes were found for restricted versus usual supply of salt/salt substitute (all P values >0.46) (Extended Data Table 3). The sensitivity analyses with imputed follow-up measurements showed similar results (Extended Data Table 3).
Supplementary analysis of the study salt supply records showed the average consumption of study salt per person per day was 11.0 ± 4.7 g (equivalent to 36.8 mmol potassium, 117.0 mmol sodium) in participants using salt substitute and 11.6 ± 3.3 g (equivalent to 0.0 mmol potassium, 199.6 mmol sodium) with usual salt (P for total weight = 0.57, P for composition < 0.001), 10.5 ± 3.7 g (equivalent to 17.3 mmol potassium, 145.9 mmol sodium) in participants with restricted supply and 12.2 ± 4.4 g (equivalent to 19.5 mmol potassium, 168.9 mmol sodium) with usual supply (P for total weight = 0.16, P for composition = 0.249).
Changes in urinary electrolytes and efficacy outcomes by randomized groups
Supplementary Table 7 gives the descriptives on the changes from the baseline to the end of intervention in 24-h urinary electrolytes and BP as well as the incidence of major cardiovascular events during the 2 years of intervention by randomized groups.
Discussion
The DECIDE-Salt trial showed clear benefits of salt substitute compared with usual salt for lowering of BP, as well as protection against cardiovascular events amongst people living in residential elderly care facilities in China. These benefits were accompanied by an increase in frequency of biochemical hyperkalemia but there was no evidence of associated adverse clinical outcomes. In contrast, efforts to restrict supply of salt/salt substitute were unsuccessful, with no detectable effect on BP during the 2 years of intervention and no benefit for cardiovascular outcomes observed.
The effect of salt substitute on BP has been shown previously but mostly in patients with HTN or high cardiovascular risk6,13 with few data for older persons or those living in residential elderly care facilities. In 2013 the Institute of Medicine called for randomized trials to examine the effects of a range of sodium levels among patients in controlled environments, such as the elderly in chronic care facilities18. Our trial contributes directly towards this goal with the majority of the study population unable to live independently and affected by multiple diseases, both cardiovascular and noncardiovascular; 74% had health conditions at baseline and about 5.3 % were bedridden.
This trial showed an effect on BP that was larger than the average effect shown in previous meta-analyses of earlier randomized trials of salt substitute among adults with or without hypertension19. The effect size on BP was also approximately double that achieved in the recently reported Salt Substitute and Stroke Study (SSaSS)13 carried out with participants with high cardiovascular risk with a history of either stroke or uncontrolled HTN but living freely in the community. Implementation of salt substitution in a collective living setting where residents have limited control over the composition of the food they eat and the seasonings they use would be expected to maximize the effect of the intervention. While the uncertainty intervals are wide, the larger BP reduction may explain the apparently greater protection against major cardiovascular events in the DECIDE-Salt trial compared with SSaSS13.
The effect on definite cardiovascular events identified in the primary analyses of salt substitute versus regular was consistent for the subsidiary analyses of all events as well as for cardiovascular death, and aligns with both the large BP reduction observed in our study and the known strong associations of BP with CVD20. Previous evidence describing the effects of salt substitute on cardiovascular events and mortality were scarce until the recent report from SSaSS13. Our data from DECIDE-Salt extend the SSaSS findings of cardiovascular protection to a broader and older population group with and without health conditions including cardiovascular and noncardiovascular disease, and with and without HTN. The DECIDE-Salt data also provide additional evidence supporting the use of salt substitute for prevention and control of HTN and its related cardiovascular consequences.
In contrast to most previous trials of salt substitute, our study sought to manage, rather than exclude, participants at risk of hyperkalemia. Typically, as people age, blood pressure increases and cardiovascular risk rises, but renal function declines21,22. Benefits from salt substitute would be expected to accrue from blood pressure reduction but poor renal function might increase the risk of hyperkalemia due to increased dietary potassium intake23. For the DECIDE-Salt study, we selected a salt substitute that contained 25% potassium chloride, which is a relatively low potassium content compared with other salt substitutes on the global market, in an effort to maximize benefit and minimize risk6. We also established a safety monitoring plan to closely monitor the risk of clinically important hyperkalemia in participants assigned to the salt substitute. With serial measures of serum potassium and access to clinical outcome data, DECIDE-Salt provided a unique opportunity to assess the safety of salt substitute, among a broad population at elevated risk. Our study showed that salt substitute right-shifts the distribution of serum potassium, causing a correspondingly increased frequency of biochemical hyperkalemia and a decreased frequency of biochemical hypokalemia. We did not detect associations of the effect of salt substitute on incidence of biochemical hyperkalemia with baseline risks for hyperkalemia such as health conditions, kidney function or other traits, although statistical power was limited. The absence of adverse clinical outcomes among those with biochemical hyperkalemia is a new observation and provides reassurance about safety. Of note, there was no clinical safety signal despite more than 70% of trial participants reporting apparent health problems at baseline, including 6% with renal disease and 8% using medications that may elevate serum potassium. These findings are also aligned with the SSaSS result, which showed no increased risk of clinical hyperkalemia events with use of salt substitute.
The observed increase in mean serum potassium and decrease in serum sodium are indicative of successful implementation and compliance of the salt substitute intervention. Quite unexpected, we found that salt substitute decreased the mean level of serum sodium, but increased risk of hyponatremia was not detected. As far as we know, this is the first study reporting the significant serum-sodium-lowering effect of salt substitute. A simple explanation is that sodium intake was reduced by the use of salt substitute. Studies have demonstrated that reduction of sodium intake would result in the reduction of serum sodium level, which may cause the BP reduction directly24. Further investigation is needed to confirm our findings.
The reasons why efforts to progressively reduce the supply of study salt/salt substitute were unsuccessful could be very complex. First, the premise of the strategy was that it would not be noticed by the participants and that no compensatory actions would be taken by participants25,26. However, site visits and analysis of self-reported data both indicated that participants were able to detect the reduction in salt use and some added nonstudy table salt to their meals. Second, the successful implementation of the strategy required a well managed salt supply system. We relied on the facility managers and cooks but some of them might not like to prepare meals with less salt. Third, this strategy is simple and incurs no cost, hence might be also taken by facilities assigned to usual supply (thus contaminating the results). The failure of the salt reduction intervention is a potentially important finding, which raises broader doubts about the feasibility of this strategy in real-world settings. In addition, the progressive nature of the intervention strategy, even if well compliant, would take 6–12 months to have an effect of more than 20% reduction in salt supply—about half of the target of the intervention. Our current analysis model did not take that nature into account. Further post hoc analysis with a more appropriate model is needed to understand the implementation of this intervention better and draw lessons from it.
The analysis was done separately for each intervention strategy and, since the effect on the restricting salt supply was not significant, no test on the interaction of two interventions was performed. This analytical strategy differed from the ‘inside the table’ analysis, in which each intervention group would be compared with the ‘pure’ control group. Our reasons included (1) the DECIDE-Salt study aimed primarily to determine the effectiveness and safety of two practical and scalable sodium reduction intervention strategies in parallel. We had no intention of testing the effect of the joint use of both intervention strategies, which is not very meaningful in practice; (2) the use of factorial design could enhance study efficiency and minimize study cost. In fact, due to funding constraints, the study was designed in such a way that there is not enough power to run the analysis in that way; and (3) many studies27,28 used the same strategy of data analysis, which is called ‘at the margins’29 analysis. We conducted this analysis under the assumption that the two interventions would act independently. We operated the study interventions independently too. If there are unrecognized interactions that might distort the results of the ‘at the margins’ analysis, the ‘inside the table’ analysis would be more appropriate but will require a much larger sample size.
The study has some important strengths. First, the collective living setting enabled a good implementation of the salt substitute intervention and complete follow-up for clinical outcome events. Second, this is one of few studies on salt substitution that did not exclude participants at risk of hyperkalemia, thus helping to define the safety of salt substitute among a broader population. DECIDE-Salt also had good statistical power to detect effects on mean serum potassium levels and the frequency of seriously deranged blood potassium levels and, while the capacity to link to adverse clinical outcomes was limited, the clinical safety findings are aligned with those observed in SSaSS13. Besides, as mentioned earlier, the use of the factorial design enhanced study efficiency and minimized the study cost by using the ‘at the margins’29 analysis method.
This study also had clear limitations. It was predominantly of men, reflecting the typical resident population in elderly care facilities in China. While the overall effect on blood pressure in DECIDE-Salt was large, the potential population-wide benefits of salt substitution for elderly Chinese may have been underestimated because the subgroup analyses indicated a larger effect in women. The collective living setting enabled implementation and robust testing of the salt substitution intervention but the scale of uptake achieved may not be generalizable to other types of settings. The planned progressive restriction of the supply of salt was not achieved as intended and did not provide a robust evaluation of this intervention strategy. Alternative analysis of the restricted supply of salt intervention, which accommodated the progressive nature of the implementation program, may have been a more appropriate analytic method. Future post hoc analysis will be undertaken to explore this possibility. The study also had many missing follow-up measurements for BP, serum potassium and urinary electrolytes. The missing data predominated in one study site, where high percentages of participants had severe illness (20%) or moved out of the facilities (17%) and where there were difficulties with study staffing. In addition, cultural factors among older Chinese discourage drawing of blood and the storage of urine during specimen collection periods. The 24-h urine collection was unwelcome also due to the complicated and time-consuming collection process and was refused by many participants. The close comparability of baseline characteristics between randomized comparisons for those who did have complete data provides some reassurance that the relative effects of intervention are unbiased. Besides, our multiple prespecified sensitivity analyses including imputation, adjustment for covariates and per-protocol assessments all produced highly comparable effect estimates. Thus, our results for the primary outcome should be considered robust and reliable. Finally, we did not fully prespecify statistical adjustment to control for the chance of false positive findings, although our primary results were robust to post hoc Bonferroni testing, and other outcomes were internally consistent and were in line with other reports in this field12,13.
In conclusion, salt substitute reduced BP and cardiovascular events in an elderly resident population. It increased the frequency of biochemical hyperkalemia but without adverse clinical outcomes. The DECIDE Trial shows net benefit from the use of salt substitute in line with data from previous trials of salt substitution showing a BP lowering effect in diverse populations30,31,32, and results of the recent SSaSS trial showing a CVD prevention effect. The studies strongly and consistently support the more widespread use of salt substitute for CVD prevention. However, efforts to restrict supply of salt failed to achieve the target for BP lowering planned in our study, which requires further analysis to better understand the feasibility and implementation of this intervention.
Methods
Project design and oversight
The design of the DECIDE-Salt study has been published previously17. The study protocol and statistical analysis plan are also published in the Supplementary Note 1. Briefly, it was a cluster-randomized, factorial trial that aimed to assess the effects of two salt reduction strategies: salt substitute and a stepwise reduction in salt supply. The rationale for using a cluster design was due mainly to the convenience to implement the interventions at cluster level but the effectiveness should be evaluated at individual level. The trial commenced in September 2017 and was carried out in 48 residential elderly care facilities, defined as the clusters in the present study, located in four regions in northern China: Xi’an city in Shaanxi province, Hohhot city in Inner Mongolia Autonomous Region, Changzhi County and Yangcheng County in Shanxi province. All regions were selected for their high sodium intake, high prevalence of HTN and history of research collaboration. The study was approved by the Peking University Institutional Review Board with a group consent obtained through discussions between the local study investigators, the administrator of each facility and the government agencies responsible for the facilities. All participants that did the baseline and follow-up surveys provided written informed consent.
Participants
To be eligible for the study, the facilities were required to (1) have 20 or more residents, either men or women determined based on self-report; (2) have staff responsible for salt supply and storage; (3) provide externally sourced food no more than once a week and (4) provide institutional agreement to participate. Given limited data about the effects of salt substitute on BP in the elderly, the study population for the intervention effects evaluation was limited to residents aged 55 years or older with BP measured at the baseline survey, expected to be resident in the facility for at least 2 years with less than 1 month’s absence each year. Residents with physician-confirmed hyperkalemia would be excluded but none met this exclusion criterion at baseline.
Randomization, study interventions and follow-up
Facilities were randomized in a 1:1:1:1 ratio to each of the factorial interventions using a central computerized process, with stratification by region. Random allocation of facilities was done after baseline survey data had been collected and by an independent statistician. Local staff responsible for the intervention implementation were aware of randomized assignment, but the outcome assessment team were masked to the allocation of the facilities.
Facilities assigned to the salt substitute group received salt substitute to replace usual salt. The salt substitute, manufactured by the China Salt General Company at Yulin in China, comprised 62.5% (mg/mg) sodium chloride, 25% (mg/mg) potassium chloride, 12.5% (mg/mg) dried food ingredient flavorings (mushroom, lemon, seaweed, hawthorn, wild jujube) and traces of amino acids. Facilities in the control group received usual salt, 100% sodium chloride, from the same company. Both salt substitute and usual salt were provided free-of-charge every 3 months for the 2-year study period in sufficient quantity to cover all cooking, seasoning and food preservation requirements.
The study salt (usual salt or salt substitute) supply to the kitchens in facilities that were allocated to the restricted supply group was reduced step by step, with the goal of achieving a facility-wide reduction of salt supply by 40% by the end of the intervention17. Responsible staff were trained to store the salt in a locked room and supply it to the kitchen on a planned schedule, with a target of reduction by 5–10% every 3 months17. During each period, the cooks can use only the salt supplied; if additional salt is requested and delivered, that amount must be documented. At the end of each stage, trained local investigators conducted a site visit to review the records on the amount of salt supplied and stored as well as the number of people living in the facility. The mean salt intake level per person was calculated periodically to assess whether the planned stepwise target had been achieved. Interviews with staff, cooks and participants followed to evaluate acceptance by participants. Once the facility successfully achieved the planned target, the next step on the salt reduction target was initiated. Otherwise, the previous planned target was retained for the next step, and the cook and responsible staff for salt supply control were retrained. During the intervention, table salt (usual salt) was allowed to help persons who had difficulty adjusting to the change. Once the goal of reduction was achieved, it was maintained until the end of the study. At the intervention kick-off event, a health education lecture was given to all residents. The emphasis was on the harms of high sodium intake, the health benefits of lower sodium intake and the plan for the stepwise salt reduction program. Posters using simple drawings and slogans were put on the walls of dining and living rooms to encourage the residents to support the sodium reduction program. At periodic monitoring visits, the study staff responsible for the intervention component reinforced the implementation messages to ensure the planned targets were achieved. The study salt supply to the kitchens in facilities that were allocated to the usual supply group was not restricted.
Follow-up was scheduled for 6, 12, 18 and 24 months after randomization. Due to the lack of human resources, follow-up visits were only done at 12 and 24 months in Xi’an. Two facilities in Xi’an dropped out before the end of study, one from the group with usual supply and usual salt and the other from the group with restricted supply and salt substitute. Both were due to administrative reasons. Local quarantine policy for the control of COVID-19 delayed final follow-up in the facilities in Yangcheng county by about 6 months but the randomized interventions were maintained throughout this period. In addition to baseline, BP measurement was sought at all visits, a blood sample at the 12- and 24-month visits and a 24-h urine sample at the 24-month visit. Inquiry about the occurrence and date of serious adverse events was done at every follow-up visit for all participants. All who were hospitalized or reported possible cardiovascular events had additional information collected for endpoint adjudication by a central committee masked to the randomized assignment. A similar process was followed for all deaths.
Safety monitoring and management
A safety monitoring and management plan was implemented for hyperkalemia17. Any participant with serum potassium >5.5 mmol l–1 detected at any timepoint was referred to a local physician for further investigation and management. Serum potassium was rechecked and an electrocardiogram performed. If a diagnosis of hyperkalemia was made, exposure to salt substitute would be discontinued until normalization of serum potassium or longer as recommended by the responsible clinician. In general, those with serum potassium >5.5 mmol l–1 or with eGFR <30 ml min–1 1.73 m–2 were considered at higher risk and were placed on a more intensive monitoring plan, which required serum potassium measurements every 3 months until two consecutive normal measurements were recorded. Participants on salt substitute were screened additionally at 3 and 6 months after the intervention initiation using a questionnaire on hyperkalemia-related signs and symptoms. Serum potassium was tested for those with positive answers to ensure safety, but those data were not used for comparison of the risk of dyskalemia between randomized comparisons.
Outcomes
The primary efficacy outcome was SBP, which was taken three times using an OMRON HEM-7136 device following American Heart Association guidelines33. Secondary efficacy outcomes were DBP, major cardiovascular events adjudicated as definite (comprising nonfatal stroke, nonfatal myocardial infarction (MI), hospitalized nonfatal heart failure or vascular death) and total mortality. Other prespecified secondary efficacy outcomes include cost-effectiveness, EQ-5D-3L (ref. 34), food satisfactoriness as well as urinary microalbumin, which will be reported separately. Prespecified safety outcomes were serum potassium, serum sodium, biochemical hyperkalemia (defined as serum potassium >5.5 mmol l–1), biochemical hyponatremia (defined as serum sodium <135 mmol l–1), and renal dysfunction (defined as eGFR <60 ml min m–2). Biochemical hypokalemia (defined as serum potassium <3.5 mmol l–1) was analyzed as exploratory safety outcome. The prespecified process indicators are 24-h urinary potassium and sodium. We also measured monitoring data on salt supply records as exploratory process indicators of salt reduction interventions.
Both serum and urinary electrolytes were measured with the ion-selective electrode method35 and serum creatinine was measured using a Roche enzymatic assay36. All assays were performed on a Roche Cobas c501 platform.
Sample size and statistical analysis
The study was designed to provide 80% statistical power (with two-sided significance level (α) = 0.05) to detect a minimum difference of 3.0 mmHg SBP between randomized comparisons17. The assumed effect size is conservative according to our previous randomized trials on salt substitute13, but it is meaningfully large for a population strategy of cardiovascular disease prevention.
The statistical analysis plan was finalized on 19 April 2021 and the database was locked on 16 May 2021. Analyses of effectiveness outcomes followed the intention-to-treat principle. We used a linear mixed model to test the intervention effects on BP, accounting for the clustering effect and adjusting for the baseline value37, among eligible participants with at least one BP measurement during follow-up (1,219 (76%) participants). The analysis was done separately for each intervention strategy in the entire randomized population (Fig. 1). It should be noted that the factorial design was employed mainly for efficiency rather than for testing of a possible interaction between interventions. We planned to test the interaction between two strategies only if both strategies were shown to be effective. Prespecified sensitivity analyses of the effects on the primary outcome included: (1) a per-protocol analysis (1,195 participants, further excluding those from one facility that discontinued the study and one facility that shifted from salt substitute to usual salt for one month); (2) multiple imputation for missing follow-up values (1,612 participants); (3) adjustment for age, sex and region (1,219 participants) and (4) exclusion of data from facilities in Xi’an (1,019 participants) where more data were missing at follow-up than in the other regions (60% in participants from Xi’an versus 9% in participants from the other regions). Notably more missing follow-up data in Xi’an was due to a high percentage of residents with severe illness (20%), a high proportion moving out of the facilities (17%) and two of four follow-up visits (6 and 18 months) not being implemented for lack of study staff in the local center.
The analyses for cardiovascular events and mortality were based on the first occurrence of each event among the 1,612 participants. Rates (100pt-yrs), HRs, 95% CIs and P values were obtained using frailty survival models with adjustment for clustering by facility38. Those who left the facilities permanently were regarded as censored and the censoring date was the date of the last day of stay. Cumulative event curves were generated using the Kaplan–Meier method39. The proportional hazard assumption was tested by Schoenfeld residuals and was not violated.
Analyses of continuous safety outcomes used a linear mixed model, accounting for the clustering effect and adjusting for the baseline value. Assessment of dichotomous safety outcomes was done by estimation from generalized linear mixed models with adjustment for clustering40. The numbers of participants with biochemical hyperkalemia followed by a cardiovascular event or death were quantified by randomized comparison group to better understand the clinical impact of biochemical hyperkalemia. The same approach as for the primary outcome was used to calculate effects on 24-h urinary potassium, sodium and microalbumin in the 639 participants with measurements.
To estimate the mean salt consumption per person per day in each facility, we first summed up the total salt supply and subtracted the remaining stored product from it, then we divided the difference by the number of people and further by the total number of days of the intervention period. The average salt consumption per person per day was defined by the sum of the mean salt consumption per person per day in each facility divided by the number of facilities in each comparison group.
The homogeneity of intervention effects on the primary outcome across participant subgroups defined by baseline characteristics (blood pressure, hypertension status, anti-HTN medication use, geographic region, sex, age and educational attainment) was tested by including interaction terms in the models and accounting for clustering. The same approach was used to test the homogeneity of intervention effects on the safety outcome across participant subgroups defined by baseline age, sex and health status as well as hyperkalemia risk status (high risk was defined as having serum potassium >5.5 mmol l–1, history of renal disease or medications that may elevate serum potassium, which include any of the following medications: ACEI/ARBs, potassium-sparing diuretics and beta-blockers).
The statistical significance level was 0.05 throughout. P values on the primary study outcomes were corrected using the Bonferroni method, that is, doubling the original P values considering that two primary outcomes were tested. This was not done for secondary outcomes; instead, we relied on the consistency of all evidence obtained from the study on different outcomes. All analyses were done using SAS v.9.4.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The availability of anonymized clinical and anthropometric data will be considered based on a proposal review subject to an internal review by the study management committee, completion of a data sharing agreement and in accordance with the Peking University’s Institutional Review Board and institutional guidelines, to ensure that the participants’ anonymity and confidentiality are protected. Please submit requests to Y.W. (wuyf@bjmu.edu.cn) copying H.L. (pucri_lihj@bjmu.edu.cn). Deidentified participant data and a data dictionary will be made available following approval. A detailed research protocol and statistical analysis plan will be shared as the supplements of this publication.
References
GBD 2017 Risk Factor Collaborators, Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1923–1994 (2018).
He, F. J., Li, J. & Macgregor, G. A. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. Br. Med. J. 346, f1325 (2013).
Filippini, T., Violi, F., D’Amico, R. & Vinceti, M. The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta-analysis. Int. J. Cardiol. 230, 127–135 (2017).
Powles, J. et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 3, e003733 (2013).
Jin, A., Xie, W. & Wu, Y. Effect of salt reduction interventions in lowering blood pressure in Chinese populations: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 10, e032941 (2020).
Hernandez, A. V. et al. Effect of low-sodium salt substitutes on blood pressure, detected hypertension, stroke and mortality. Heart 105, 953–960 (2019).
O’Donnell, M. J. et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA 306, 2229–2238 (2011).
Dent, A., Walmsley, D. & Dhandapani, S. Hyperkalaemia is a risk with low sodium salt in vulnerable patients. Br. Med. J. 343, d4514 (2011).
Ayach, T., Nappo, R. W., Paugh-Miller, J. L. & Ross, E. A. Life-threatening hyperkalemia in a patient with normal renal function. Clin. Kidney J. 7, 49–52 (2014).
Greer, R. C. et al. Potassium-enriched salt substitutes as a means to lower blood pressure: benefits and risks. Hypertension 75, 266–274 (2020).
Marklund, M. et al. Estimated population wide benefits and risks in China of lowering sodium through potassium enriched salt substitution: modelling study. Br. Med. J. 369, m824 (2020).
Chang, H. Y. et al. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am. J. Clin. Nutr. 83, 1289–1296 (2006).
Neal, B. et al. Effect of salt substitution on cardiovascular events and death. N. Engl. J. Med. 385, 1067–1077 (2021).
Losby, J. L. et al. Sodium-reduction strategies for meals prepared for older adults. J. Public Health Manag. Pract. 20, S23–S30 (2014).
Rodgers, A. & Neal, B. Less salt does not necessarily mean less taste. Lancet 353, 1332 (1999).
Girgis, S. et al. A one-quarter reduction in the salt content of bread can be made without detection. Eur. J. Clin. Nutr. 57, 616–620 (2003).
Jin, A. et al. Impact of salt substitute and stepwise reduction of salt supply on blood pressure in residents in senior residential facilities: design and rationale of the DECIDE-Salt trial. Am. Heart J. 226, 198–205 (2020).
Institute of Medicine. Sodium Intake in Populations: Assessment of Evidence (eds. Strom, B.L., Yaktine, A.L. & Oria, M.) 1–10 (The National Academies Press, 2013).
Peng, Y. G. et al. Effects of salt substitutes on blood pressure: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 100, 1448–1454 (2014).
Ettehad, D. et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 387, 957–967 (2016).
Eriksen, B. O. et al. GFR in healthy aging: an individual participant data meta-analysis of iohexol clearance in European population-based cohorts. J. Am. Soc. Nephrology 31, 1602–1615 (2020).
Liu, P. et al. Accounting for age in the definition of chronic kidney disease. JAMA Intern. Med. 181, 1359–1366 (2021).
Jin, A. et al. Normal range of serum potassium, prevalence of dyskalaemia and associated factors in Chinese older adults: a cross-sectional study. BMJ Open 10, e039472 (2020).
He, F. J., Markandu, N. D., Sagnella, G. A., de Wardener, H. E. & MacGregor, G. A. Plasma sodium: ignored and underestimated. Hypertension 45, 98–102 (2005).
Bolhuis, D. P. et al. A salt reduction of 50% in bread does not decrease bread consumption or increase sodium intake by the choice of sandwich fillings. J. Nutr. 141, 2249–2255 (2011).
McGuire, S. Institute of Medicine. 2010. Strategies to reduce sodium intake in the United States. Washington, DC: The National Academies Press. Adv. Nutr. 1, 49–50 (2010).
Manson, J. E. et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N. Engl. J. Med. 380, 23–32 (2019).
Manson, J. E. et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 380, 33–44 (2019).
McAlister FA, S. S., Sackett, D. L. & Altman, D. G. Analysis and reporting of factorial trials: a systematic review. JAMA 289, 2545–2553 (2003).
Bernabe-Ortiz, A. et al. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nat. Med. 26, 374–378 (2020).
Yu, J. et al. Effects of a reduced-sodium added-potassium salt substitute on blood pressure in rural Indian hypertensive patients: a randomized, double-blind, controlled trial. Am. J. Clin. Nutr. 114, 185–193 (2021).
Geleijnse, J. M., Witteman, J. C., Bak, A. A., den Breeijen, J. H. & Grobbee, D. E. Reduction in blood pressure with a low sodium, high potassium, high magnesium salt in older subjects with mild to moderate hypertension. BMJ 309, 436–440 (1994).
Muntner, P. et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension 73, e35–e66 (2019).
Rabin, R. & de Charro, F. EQ-5D: a measure of health status from the EuroQol Group. Ann. Med. 33, 337–343 (2001).
Nikolac Gabaj, N. et al. Precision, accuracy, cross reactivity and comparability of serum indices measurement on Abbott Architect c8000, Beckman Coulter AU5800 and Roche Cobas 6000 c501 clinical chemistry analyzers. Clin. Chem. Lab. Med. 56, 776–788 (2018).
Carobene, A., Ceriotti, F., Infusino, I., Frusciante, E. & Panteghini, M. Evaluation of the impact of standardization process on the quality of serum creatinine determination in Italian laboratories. Clin. Chim. Acta 427, 100–106 (2014).
Cnaan, A., Laird, N. M. & Slasor, P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat. Med. 16, 2349–2380 (1997).
Hougaard, P. Frailty models for survival data. Lifetime Data Anal. 1, 255–273 (1995).
Goel, M. K., Khanna, P. & Kishore, J. Understanding survival analysis: Kaplan–Meier estimate. Int. J. Ayurveda Res. 1, 274–278 (2010).
Tango, T. On the repeated measures designs and sample sizes for randomized controlled trials. Biostatistics 17, 334–349 (2016).
Acknowledgements
The authors thank all facility residents for their participation and cooperation. We also like to thank all investigators, study team members, facility managers and staff, and administrative agencies for their proactive participation in the study. We are also grateful to K. Liu at the Northwestern University School of Medicine for his advice on study design and statistical analysis plan development. The trial was supported by the National Key Research and Development Program, Ministry of Science and Technology of China, through the research grant ‘Diet, ExerCIse and CarDiovascular hEalth (DECIDE) project’ (2016YFC1300200). China Salt General Company at Yulin provided the usual salt and salt substitute used in the study free of charge. The funders of the study had no role in the study design, data collection, data analysis, data interpretation or writing of the report.
Author information
Authors and Affiliations
Contributions
Y.W. and A.J. designed the study with advice from D.L. and B.N. The Statistical Analysis Plan was finalized by Y.W., P.G. and Y.Y. before closing of the database. Y.Y. and A.J. analyzed and verified the data analysis. P.G., D.L. and P.E. helped on data analysis and interpretation. Y.Y., A.J., B.N. and Y.W. wrote the first draft with all coauthors participating in the subsequent reviews and revisions. Y.W. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
B.N. is supported by a National Health and Medical Research Council Investigator Grant (APP1197709). P.E. is director of the UK Medical Research Council (MRC) Centre for Environment and Health (MR/L01341X/1; MR/S019669/1) and acknowledges support from the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre, the Imperial College British Heart Foundation Centre for Research Excellence (RE/18/4/34215) and the UK Dementia Research Institute at Imperial College London (MC_PC_17114).
Peer review
Peer review information
Nature Medicine thanks Victor Volovici, Simon Capewell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Michael Basson, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Effects on overall follow-up systolic blood pressure of salt substitute versus usual salt in participant subgroups.
Mean difference and its 95%CI of the effect on overall follow-up systolic blood pressure of salt substitute versus usual salt in participant subgroups was obtained by linear mixed model, accounting for the clustering effect and adjusting for the baseline value. The p value was two-sided and was not adjusted for multiple comparison.
Extended Data Fig. 2 Effects on overall follow-up systolic blood pressure of restricted supply versus usual supply in participant subgroups.
Mean difference and its 95%CI of the effect on overall follow-up systolic blood pressure of restricted supply versus usual supply in participant subgroups was obtained by linear mixed model, accounting for the clustering effect and adjusting for the baseline value. The p value was two-sided and was not adjusted for multiple comparison.
Extended Data Fig. 3 Impact on hyperkalaemia of salt substitute versus usual salt in participant subgroups by selected baseline characteristics.
The error bar is the risk ratio(RR) and its 95% confidence interval of hyperkalemia for salt substitute versus usual salt in each subgroup, obtained by generalized linear mixed models with adjustment for clustering. The p value was two-sided and was not adjusted for multiple comparison. Health conditions included any of the following: hypertension, diabetes mellitus, coronary heart disease, stroke, chronic kidney disease, cancer, chronic obstructive pulmonary disease or being bedridden. Risk of hyperkalemia was defined high if the participant met any of the following at baseline: serum potassium > 5.5 mmol/l, using any medication that may elevate potassium (ACEI/ARBs, potassium-sparing diuretics, beta-blockers), with history of renal disease and eGFR < 60 ml/min*1.73 m3.
Supplementary information
Supplementary Information
Supplementary Tables 1–7.
Supplementary Data
Study protocols and amendments, statistical analysis plan and amendments and original materials in Chinese.
Supplementary Note
Research group information.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, Y., Jin, A., Neal, B. et al. Salt substitution and salt-supply restriction for lowering blood pressure in elderly care facilities: a cluster-randomized trial. Nat Med 29, 973–981 (2023). https://doi.org/10.1038/s41591-023-02286-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02286-8
This article is cited by
-
Experience with 2 years’ intervention to progressively reduce salt supply to kitchens in elderly care facilities—challenges and further research: post hoc analysis of the DECIDE-Salt randomized clinical trial
BMC Medicine (2023)
-
Benefits of salt substitution in care facilities for the elderly
Nature Medicine (2023)