Abstract
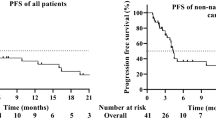
Anti-programmed cell death protein 1 (PD-1) therapy is a standard of care in recurrent metastatic head and neck squamous cell carcinoma (RMHNSCC). Vascular endothelial growth factor inhibitors, including tyrosine kinase inhibitors, have immunomodulatory properties and have offered promising results when combined with anti-PD-1 agents. We conducted a phase 2, multicenter, single-arm trial of pembrolizumab and cabozantinib in patients with RMHNSCC who had Response Evaluation Criteria in Solid Tumors v.1.1 measurable disease and no contraindications to either agent. We assessed the primary end points of tolerability and overall response rate to the combination with secondary end points of progression-free survival and overall survival and performed correlative studies with PDL-1 and combined positive score, CD8+ T cell infiltration and tumor mutational burden. A total of 50 patients were screened and 36 were enrolled with 33 evaluable for response. The primary end point was met, with 17 out of 33 patients having a partial response (52%) and 13 (39%) stable disease with an overall clinical benefit rate of 91%. Median and 1-year overall survival were 22.3 months (95% confidence interval (CI) = 11.7–32.9) and 68.4% (95% CI = 45.1%–83.5%), respectively. Median and 1-year progression-free survival were 14.6 months (95% CI = 8.2–19.6) and 54% (95% CI = 31.5%–72%), respectively. Grade 3 or higher treatment-related adverse events included increased aspartate aminotransferase (n = 2, 5.6%). In 16 patients (44.4%), the dose of cabozantinib was reduced to 20 mg daily. The overall response rate correlated positively with baseline CD8+ T cell infiltration. There was no observed correlation between tumor mutational burden and clinical outcome. Pembrolizumab and cabozantinib were well tolerated and showed promising clinical activity in patients with RMHNSCC. Further investigation of similar combinations are needed in RMHNSCC. The trial is registered at ClinicalTrials.gov under registration no. NCT03468218.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon written request and clarification from the requesting party as to how the data will be used. Requests for data sharing will be responded to within 2 weeks. The WES data has been submitted to the SRA. The full study protocol is available as SupplementaryInformation.
References
Mody, M. D., Rocco, J. W., Yom, S. S., Haddad, R. I. & Saba, N. F. Head and neck cancer. Lancet 398, 2289–2299 (2021).
Vermorken, J. B. et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 359, 1116–1127 (2008).
Eberhardt, C. S. et al. Functional HPV-specific PD-1+ stem-like CD8 T cells in head and neck cancer. Nature 597, 279–284 (2021).
Wieland, A. et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 597, 274–278 (2021).
Ferris, R. L. et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 375, 1856–1867 (2016).
Burtness, B. et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394, 1915–1928 (2019).
Saba, N. F. et al. Nivolumab versus investigator’s choice in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: efficacy and safety in CheckMate 141 by age. Oral Oncol. 96, 7–14 (2019).
Harrington, K. J. et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 18, 1104–1115 (2017).
Saba, N. F. et al. Targeting angiogenesis in squamous cell carcinoma of the head and neck: opportunities in the immunotherapy era. Cancers 14, 1202 (2022).
Li, Y.-L., Zhao, H., & Ren, X.-B. Relationship of VEGF/VEGFR with immune and cancer cells: staggering or forward? Cancer Biol. Med. 13, 206–214 (2016).
Peeters, M. J. W., Rahbech, A., & Thor Straten, P. TAM-ing T cells in the tumor microenvironment: implications for TAM receptor targeting. Cancer Immunol. Immunother. 69, 237–244 (2020).
George, D. J. et al. Cabozantinib versus sunitinib for untreated patients with advanced renal cell carcinoma of intermediate or poor risk: subgroup analysis of the alliance A031203 CABOSUN trial. Oncologist 24, 1497–1501 (2019).
Abou-Alfa, G. K., Borgman-Hagey, A. E. & Kelley, R. K. Cabozantinib in hepatocellular carcinoma. N. Engl. J. Med. 379, 1384–1385 (2018).
Stukalin, I. et al. Real-world outcomes of nivolumab and cabozantinib in metastatic renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Curr. Oncol. 26, e175–e179 (2019).
Brose, M. S. et al. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 22, 1126–1138 (2021).
Chen, J.-Y. et al. Cancer-derived VEGF-C increases chemokine production in lymphatic endothelial cells to promote CXCR2-dependent cancer invasion and MDSC recruitment. Cancers 11, 1120 (2019).
Linger, R. M., Keating, A. K., Earp, H. S. & Graham, D. K. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv. Cancer Res. 100, 35–83 (2008).
Grüllich, C. Cabozantinib: multi-kinase inhibitor of MET, AXL, RET, and VEGFR2. Recent Results Cancer Res. 211, 67–75 (2018).
Desai, A. & Small, E. J. Treatment of advanced renal cell carcinoma patients with cabozantinib, an oral multityrosine kinase inhibitor of MET, AXL and VEGF receptors. Future Oncol. 15, 2337–2348 (2019).
Wu, H. et al. Mer regulates microglial/macrophage M1/M2 polarization and alleviates neuroinflammation following traumatic brain injury. J. Neuroinflammation 18, 2 (2021).
Bergerot, P., Lamb, P., Wang, E. & Pal, S. K. Cabozantinib in combination with immunotherapy for advanced renal cell carcinoma and urothelial carcinoma: rationale and clinical evidence. Mol. Cancer Ther. 18, 2185–2193 (2019).
Chalmers, Z. R. et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 9, 34 (2017).
Choueiri, T. K. et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 384, 829–841 (2021).
Jiang, A.-M. et al. Tumor mutation burden, immune cell infiltration, and construction of immune-related genes prognostic model in head and neck cancer. Int. J. Med. Sci. 18, 226–238 (2021).
Li, J. et al. Tumor cell-intrinsic factors underlie heterogeneity of immune cell infiltration and response to immunotherapy. Immunity 49, 178–193 (2018).
Liu, Z. et al. TIGIT and PD-1 expression atlas predicts response to adjuvant chemotherapy and PD-L1 blockade in muscle-invasive bladder cancer. Br. J. Cancer 126, 1310–1317 (2022).
Du, H. et al. The co-expression characteristics of LAG3 and PD-1 on the T cells of patients with breast cancer reveal a new therapeutic strategy. Int. Immunopharmacol. 78, 106113 (2020).
van Boxtel, W. et al. Excessive toxicity of cabozantinib in a phase II study in patients with recurrent and/or metastatic salivary gland cancer. Eur. J. Cancer 161, 128–137 (2022).
Emancipator, K. et al. Comparing programmed death ligand 1 scores for predicting pembrolizumab efficacy in head and neck cancer. Mod. Pathol. 34, 532–541 (2021).
Xiong, Y. et al. Nck-associated protein 1 associates with HSP90 to drive metastasis in human non-small-cell lung cancer. J. Exp. Clin. Cancer Res. 38, 122 (2019).
Zhao, X. et al. FGFR4 provides the conduit to facilitate FGF19 signaling in breast cancer progression. Mol. Carcinog. 57, 1616–1625 (2018).
Chen, X. et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 32, 1220–1222 (2016).
Kim, S. et al. Strelka2: fast and accurate calling of germline and somatic variants. Nat. Methods 15, 591–594 (2018).
Knaus, B. J. & Grünwald, N. J. vcfr: a package to manipulate and visualize variant call format data in R. Mol. Ecol. Resour. 17, 44–53 (2017).
Bauml, J. et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J. Clin. Oncol. 35, 1542–1549 (2017).
Zhou, H., Lee, J. J. & Yuan, Y. BOP2: Bayesian optimal design for phase II clinical trials with simple and complex endpoints. Stat. Med. 36, 3302–3314 (2017).
Liu, N., Zhou, Y. & Lee, J. J. IPDfromKM: reconstruct individual patient data from published Kaplan–Meier survival curves. BMC Med. Res. Methodol. 21, 111 (2021).
Schober, P. & Vetter, T. R. Chi-square tests in medical research. Anesth. Analg. 129, 1193 (2019).
Hu, K. & Tong, W. Which to select when evaluating risk factors for permanent stoma, COX regression model or logistic regression model? Ann. Transl. Med. 9, 1634 (2021).
Acknowledgements
This research was supported by a grant from Exelixis to N.F.S. The research reported in this publication was supported in part by the Biostatistics Shared Resource of Winship Cancer Institute of Emory University and National Institutes of Health (IH)/NCI under award no. P30CA138292. The computational resources used were partially funded by the NIH Shared Instrumentation grant no. 1S10OD021644-01A1; this research was partially supported by a Huntsman Cancer Institute at the University of Utah Cancer Center Support grant no. P30CA042014, the National Institute of Dental and Craniofacial Research (no. R01DE030508 to A.C.T. and C.H.C.) and the James and Esther King Biomedical Research grant (no. 21K04 to C.H.C. and A.C.T.). The biomarker study was partially supported by a Winship Invest$ Team Science Award (to Y.T. and N.F.S.), I3 Nexus award from Emory School of Medicine (to Y.T. and N.F.S.) and the National Institute of Dental and Craniofacial Research grant no. R01DE028351 (to Y.T.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors thank A. Hammond for her editorial feedback.
Author information
Authors and Affiliations
Contributions
N.F.S. conceived, designed and implemented the study, wrote the paper and analyzed the generated data. A.E. collected the data. A.M.-V. and Y.L. performed the statistical analysis. K.M. performed the PD-L1 and p16 testing. M.A. collected and analyzed the data. A.C.T., M.Z.H.F. and R.C. performed the WES and TMB calculations. C.E.S., M.P., N.C.S., W.S., J.E.B., S.R., J.R., M.M., J.M., K.K. and D.M.S. enrolled and treated patients on the study, collected the data and reviewed the paper. G.Z.H. and Y.T. performed the intratumoral CD8+ analysis. C.H.C. implemented the study at Moffitt Cancer Center, collected, and analyzed the data and reviewed the paper. All authors contributed to the revision of the paper.
Corresponding author
Ethics declarations
Competing interests
N.F.S. reports advisory roles for Merck, AZ, Eisai, Exelixis, Vaccinex, BNT and CUE. J.E.B. reports advisory roles for Galera Therapeutics and Castle Biosciences. N.C.S. reports research funding from Astex. C.H.C. reports advisory board participation for Merck, Exelixis, Fulgent, Genmab and Brooklyn ImmunoTherapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Vivian W.Y. Lui and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ulrike Harjes, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Survival outcomes by T and N stage.
Overall survival by T (a) and N (b) stage (p=0.891, p=0.550 respectively), and progression-free survival by T (c) and N (d) stage (p=0.599, p=0.732 respectively).
Extended Data Fig. 2 Survival outcomes by response and smoking history.
PFS (a) and OS (b) in responders versus non-responders (p=0.028 and p=0.3347 respectively). (c) OS by smoking history (former or current smokers versus never smokers) (p=0.442).
Extended Data Fig. 3 Tumor mutational burdens for HNSCC patients.
Distribution of the TMB (Mut/MB) computed for the 16 HNSCC patients with sufficient tumor sample. The red line indicates the median of TMB for these patients, which is 6.71/MB.
Extended Data Fig. 4 Survival outcomes by tumor mutation burden.
(a) Overall survival and (b) Progression-free survival by tumor mutation burden (TMB).
Supplementary information
Supplementary Information
Clinical trial protocol.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saba, N.F., Steuer, C.E., Ekpenyong, A. et al. Pembrolizumab and cabozantinib in recurrent metastatic head and neck squamous cell carcinoma: a phase 2 trial. Nat Med 29, 880–887 (2023). https://doi.org/10.1038/s41591-023-02275-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02275-x
This article is cited by
-
Combined IL6 and CCR2 blockade potentiates antitumor activity of NK cells in HPV-negative head and neck cancer
Journal of Experimental & Clinical Cancer Research (2024)
-
Tumor mutational burden predictability in head and neck squamous cell carcinoma patients treated with immunotherapy: systematic review and meta-analysis
Journal of Translational Medicine (2024)
-
New clinical trial design in precision medicine: discovery, development and direction
Signal Transduction and Targeted Therapy (2024)
-
Exploring the crosstalk between endothelial cells, immune cells, and immune checkpoints in the tumor microenvironment: new insights and therapeutic implications
Cell Death & Disease (2023)
-
Current systemic treatment options and new developments in palliative first-line treatment of head and neck squamous cell carcinoma
memo - Magazine of European Medical Oncology (2023)