Abstract
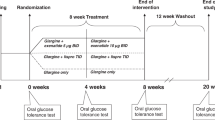
Hyperglucagonemia contributes to hyperglycemia in patients with type 1 diabetes (T1D); however, novel therapeutics that block glucagon action could improve glycemic control. This phase 2 study evaluated the safety and efficacy of volagidemab, an antagonistic monoclonal glucagon receptor (GCGR) antibody, as an adjunct to insulin therapy in adults with T1D. The primary endpoint was change in daily insulin use at week 12. Secondary endpoints included changes in hemoglobin A1c (HbA1c) at week 13, in average daily blood glucose concentration and time within target range as assessed by continuous blood glucose monitoring (CGM) and seven-point glucose profile at week 12, incidence of hypoglycemic events, the proportion of subjects who achieve HbA1c reduction of ≥0.4%, volagidemab drug concentrations and incidence of anti-drug antibodies. Eligible participants (n = 79) were randomized to receive weekly subcutaneous injections of placebo, 35 mg volagidemab or 70 mg volagidemab. Volagidemab produced a reduction in total daily insulin use at week 12 (35 mg volagidemab: −7.59 units (U) (95% confidence interval (CI) −11.79, −3.39; P = 0.040 versus placebo); 70 mg volagidemab: −6.64 U (95% CI −10.99, −2.29; P = 0.084 versus placebo); placebo: −1.27 U (95% CI −5.4, 2.9)) without meeting the prespecified significance level (P < 0.025). At week 13, the placebo-corrected reduction in HbA1c percentage was −0.53 (95% CI −0.89 to −0.17, nominal P = 0.004) in the 35 mg volagidemab group and −0.49 (95% CI −0.85 to −0.12, nominal P = 0.010) in the 70 mg volagidemab group. No increase in hypoglycemia was observed with volagidemab therapy; however, increases in serum transaminases, low-density lipoprotein (LDL)-cholesterol and blood pressure were observed. Although the primary endpoint did not meet the prespecified significance level, we believe that the observed reduction in HbA1c and tolerable safety profile provide a rationale for further randomized studies to define the long-term efficacy and safety of volagidemab in patients with T1D. See clinicaltrials.gov registration no. NCT03117998.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data from these analyses cannot be made publicly available due to the sponsor’s contractual obligations. We encourage researchers or parties interested in collaboration for noncommercial use to apply to the corresponding author. Applications should outline specifically what data they are interested in receiving and how the data will be used. All data shared will be de-identified and will be made available 2 years after the date of publication. A signed data access agreement with the sponsor is required before accessing the shared data. Source data are provided with this paper.
Change history
17 March 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41591-023-02301-y
References
Janah, L. et al. Glucagon receptor signaling and glucagon resistance. Int J. Mol. Sci. 20, 3314 (2019).
Fredheim, S. et al. The influence of glucagon on postprandial hyperglycaemia in children 5 years after onset of type 1 diabetes. Diabetologia 58, 828–834 (2015).
Gelling, R. W. et al. Lower blood glucose, hyperglucagonemia, and pancreatic α cell hyperplasia in glucagon receptor knockout mice. Proc. Natl Acad. Sci. USA 100, 1438–1443 (2003).
Wang, M. et al. Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway. Proc. Natl Acad. Sci. USA 112, 2503–2508 (2015).
Gu, W. et al. Long-term inhibition of the glucagon receptor with a monoclonal antibody in mice causes sustained improvement, with reversible alpha-cell hyperplasia and hyperglucagonemia. J. Pharm. Exp. Ther. 331, 871–881 (2009).
Yan, H. et al. Fully human monoclonal antibodies antagonizing the glucagon receptor improve glucose homeostasis in mice and monkeys. Pharm. Exp. Ther. 329, 102–111 (2009).
Pettus, J. et al. Effect of a glucagon receptor antibody (REMD-477) in type 1 diabetes: a randomized controlled trial. Diabetes Obes. Metab. 20, 1302–1305 (2018).
Althunian, T. A. et al. Assessment of the regulatory dialogue between pharmaceutical companies and the European Medicines Agency on the choice of noninferiority margins. Clin. Ther. 42, 1588–1594 (2020).
Navarro, V. & Senior, J. Drug-related hepatotoxicity. N. Engl. J. Med. 354, 731–739 (2006).
Van der Schueren, B. et al. Obesity in people living with type 1 diabetes. Lancet Diabetes Endocrinol. 9, 776–785 (2021).
Gregory, J. M. et al. Iatrogenic hyperinsulinemia, not hyperglycemia, drives insulin resistance in type 1 diabetes as revealed by comparison with GCK-MODY (MODY2). Diabetes 68, 1565–1576 (2019).
Arcaro, G. et al.Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation 105, 576–582 (2002).
Unger, R. H. & Cherrington, A. D. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J. Clin. Invest. 122, 4–12 (2012).
Wang, M. Y. et al. Glucagon blockade restores functional β-cell mass in type 1 diabetic mice and enhances function of human islets. Proc. Natl Acad. Sci. USA 118, e2022142118 (2021).
Levetan, C. & Pierce, S. Distinctions between the islets of mice and men: implications for new therapies for type 1 and 2 diabetes. Endocr. Pr. 19, 301–312 (2013).
Cabrera, O. et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl Acad. Sci. USA 103, 2334–2339 (2006).
Brissova, M. et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J. Histochem. Cytochem. 53, 1087–1097 (2005).
Holt, R. et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 44, 2589–2625 (2021).
Garg, S. et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N. Engl. J. Med. 377, 2337–2348 (2017).
Dandona, P. et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 5, 864–876 (2017).
Mathieu, C. et al. Efficacy and safety of liraglutide added to insulin treatment in type 1 diabetes: the ADJUNCT ONE treat-to-target randomized trial. Diabetes Care 39, 1702–1710 (2016).
Kazda, C. et al. Evaluation of efficacy and safety of the glucagon receptor antagonist LY2409021 in patients with type 2 diabetes: 12- and 24-week phase 2 studies. Diabetes Care 39, 1241–1249 (2016).
Guzman, C. et al. Treatment with LY2409021, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Diabetes Obes. Metab. 19, 1521–1528 (2017).
Engel, S. et al. Efficacy and tolerability of MK-0893, a glucagon receptor antagonist (GRA), in patients with type 2 diabetes (T2DM). Diabetes 60, A85 (2011).
Guan, H. et al. Glucagon receptor antagonism induces increased cholesterol absorption. J. Lipid Res. 56, 2183–2195 (2015).
Kazda, C. et al. Treatment with the glucagon receptor antagonist LY2409021 increases ambulatory blood pressure in patients with type 2 diabetes. Diabetes Obes. Metab. 19, 1071–1077 (2017).
Murtagh, J., Binnion, P., Lal, S., Hutchison, K. & Fletcher, E. Haemodynamic effects of glucagon. Br. Hear. J. 32, 307–315 (1970).
Acknowledgements
This study was sponsored and funded by REMD Biotherapeutics. REMD Biotherapeutics was directly involved in the design, conduct, analysis and reporting of the study. Research reported in this publication was also supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R44DK108305. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Medical writing support, under the direction of the authors, was provided by J. Leonoudakis and was funded by REMD Biotherapeutics in accordance with Good Publication Practice guidelines.
Author information
Authors and Affiliations
Contributions
All authors contributed to study concept and study supervision. E.D.B, R.X., H.Y. and D.T. were responsible for study administration. J.P., S.C.B., M.P.C., D.S.D., T.S.B., H.K.A., L.J.K., J.R., M.H.M.C., B.W.B., E.D.B., S.K.G. and S.K. provided patients and conducted the investigation. J.P. and D.T. wrote the original draft of the manuscript. All authors critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.P. served as an advisor to Novo Nordisk and Sanofi; and served as a consultant to Diasome Pharmaceuticals, Insulet Corporation, Lilly Diabetes, MannKind Corporation and Tandem Diabetes Care. S.C.B. served as a consultant to Cecelia Health and received research support from Dexcom. M.P.C. received research support from Abbott Diabetes, Biolinq, Dexcom, Eli Lilly and Company, Medtronic, Merck & Co., Novo Nordisk A/S, Pfizer and Viacyte. T.S.B. served as a consultant to Abbott, Lifescan, Mannkind, Medtronic, Novo and Sanofi; received research support from Abbott Diabetes, Abbott Rapid Diagnostics, Biolinq, Capillary Biomedical, Dexcom, Eli Lilly, Kowa, Lexicon, Livongo, Mannkind, Medtronic, Novo Nordisk, REMD, Sanofi, Sanvita, Senseonics, Viacyte, vTv Therapeutics and Zealand Pharma; and served as a speaker for BD, Medtronic and Sanofi. H.K.A. served as a consultant to the American Diabetes Association; received research support from Dexcom, Eli Lilly and Company, IM Therapeutics, MannKind Corporation, REMD Biotherapeutics and Senseonics; and served as a speaker for the American Diabetes Association. L.J.K. received research support from Abbott Diabetes, Dong-A ST Co. Ltd., Gan & Lee Pharmaceuticals, Lexicon Pharmaceuticals, Lilly Diabetes, Medtronic, Novo Nordisk, Oramed Pharmaceuticals, Pfizer and REMD Biotherapeutics. J.R. served as a board member for Applied Therapeutics, Boehringer Ingelheim Pharmaceuticals, Eli Lilly and Company, Intarcia Therapeutics, Novo Nordisk, Oramed Pharmaceuticals and Sanofi; and served as a consultant to Applied Therapeutics, Boehringer Ingelheim Pharmaceuticals, Eli Lilly and Company, Intarcia Therapeutics and Novo Nordisk. B.W.B. served as an advisor to Eli Lilly and Company; served as a consultant to Bigfoot Biomedical, Companion Medical, Lexicon Pharmaceuticals, Medtronic, Novo Nordisk and Zealand Pharma A/S; received research support from Abvance Therapeutics, Dexcom, Diasome Pharmaceuticals, Dompe, Eli Lilly and Company, Eyenuk, Insulet Corporation, Jaeb Center for Health Research, Medtronic, Nova Biomedical, Novo Nordisk, Provention Bio, REMD Biotherapeutics, Sanofi, Senseonics, Viacyte, vTv Therapeutics and Xeris Pharmaceuticals; served as a speaker for AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Eli Lilly and Company, MannKind Corporation, Medtronic, Novo Nordisk and Sanofi; and is a stock/shareholder in AgaMatrix, Aseko and Glytec, LLC. E.D.B. served as a consultant to REMD Biotherapeutics. R.X. and H.Y. served as board members for REMD Biotherapeutics. D.T. is an employee for REMD Biotherapeutics. S.K.G. served as an advisor to Eli Lilly and Company, Medtronic, Novo Nordisk and Zealand Pharma A/S; and received research support from Dexcom, Eli Lilly and Company, and Medtronic. S.K. served as an advisor to Altimmune, Janssen and ProSciento, and received research support from Janssen Research & Development, LLC. D.S.D. and M.G.M.C have no competing interests to disclose.
Peer review
Peer review information
Nature Medicine thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Jennifer Sargent, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Blood glucose concentrations from continuous glucose monitoring.
Blood glucose concentration data from available continuous glucose monitoring data are presented as least square means with error bars representing 95% confidence intervals for participants treated with placebo (n = 27), 35 mg volagidemab (n = 26), or 70 mg volagidemab (n = 25). Change in a, average daily glucose; b, percent of samples in target range (70–180 mg/dL); c, percent of samples below target range (<70 mg/dL); d, percent of samples below target range (<55 mg/dL); e, percent samples above target range (>180 mg/dL).
Extended Data Fig. 2 Proportion of patients who reached target hemoglobin A1c ≤ 7.0.
The proportion of patients who reached a target HbA1c level of ≤7.0 are presented for participants treated with placebo (n = 27), 35 mg volagidemab (n = 26), or 70 mg volagidemab (n = 25). Error bars represent 95% confidence intervals.
Extended Data Fig. 3 Adverse events of special interest.
Adverse event data are presented as arithmetic means with error bars representing 95% confidence intervals for participants treated with placebo (n = 27), 35 mg volagidemab (n = 26), or 70 mg volagidemab (n = 26). Change in a, serum alanine aminotransferase (ALT); b, serum aspartate aminotransferase (AST); c, low-density lipoprotein (LDL) cholesterol; d, systolic blood pressure (SBP); and e, diastolic blood pressure (DBP).
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pettus, J., Boeder, S.C., Christiansen, M.P. et al. Glucagon receptor antagonist volagidemab in type 1 diabetes: a 12-week, randomized, double-blind, phase 2 trial. Nat Med 28, 2092–2099 (2022). https://doi.org/10.1038/s41591-022-02011-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-022-02011-x
This article is cited by
-
G protein-coupled receptors (GPCRs): advances in structures, mechanisms, and drug discovery
Signal Transduction and Targeted Therapy (2024)
-
GLP-1 metabolite GLP-1(9–36) is a systemic inhibitor of mouse and human pancreatic islet glucagon secretion
Diabetologia (2024)
-
Structure, function and drug discovery of GPCR signaling
Molecular Biomedicine (2023)
-
Revisiting the role of glucagon in health, diabetes mellitus and other metabolic diseases
Nature Reviews Endocrinology (2023)
-
Metabolic Messengers: glucagon
Nature Metabolism (2023)