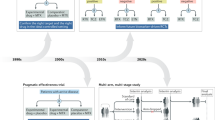
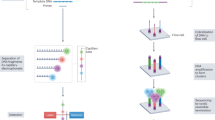
Abstract
Systemic autoimmune rheumatic diseases (SARDs) exhibit extensive heterogeneity in clinical presentation, disease course, and treatment response. Therefore, precision medicine — whereby treatment is tailored according to the underlying pathogenic mechanisms of an individual patient at a specific time — represents the ‘holy grail’ in SARD clinical care. Current strategies include treat-to-target therapies and autoantibody testing for patient stratification; however, these are far from optimal. Recent innovations in high-throughput ‘omic’ technologies are now enabling comprehensive profiling at multiple levels, helping to identify subgroups of patients who may taper off potentially toxic medications or better respond to current molecular targeted therapies. Such advances may help to optimize outcomes and identify new pathways for treatment, but there are many challenges along the path towards clinical translation. In this Review, we discuss recent efforts to dissect cellular and molecular heterogeneity across multiple SARDs and future directions for implementing stratification approaches for SARD treatment in the clinic.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rosenblum, M. D., Gratz, I. K., Paw, J. S. & Abbas, A. K. Treating human autoimmunity: current practice and future prospects. Sci. Transl. Med. 4, 125sr1 (2012).
Pitzalis, C., Choy, E. H. S. & Buch, M. H. Transforming clinical trials in rheumatology: towards patient-centric precision medicine. Nat. Rev. Rheumatol. 16, 590–599 (2020).
Kim, N., Eum, H. H. & Lee, H.-O. Clinical perspectives of single-cell RNA sequencing. Biomolecules 11, 1161 (2021).
Cocco, E., Scaltriti, M. & Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 15, 731–747 (2018).
Manthiram, K., Zhou, Q., Aksentijevich, I. & Kastner, D. L. The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation. Nat. Immunol. 18, 832–842 (2017).
Dalbeth, N. et al. Gout. Nat. Rev. Dis. Prim. 5, 69 (2019).
Singh, J. A. et al. 2012 Update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 64, 625–639 (2012).
Navarra, S. V. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 377, 721–731 (2011).
Furie, R. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheumatol. 63, 3918–3930 (2011).
Fernando, M. M. A. & Isenberg, D. A. How to monitor SLE in routine clinical practice. Ann. Rheumatol. Dis. 64, 524 (2005).
Merrill, J. T. et al. Phase 2 trial of iberdomide in systemic lupus erythematosus. N. Engl. J. Med. 386, 1034–1045 (2022).
Lakhanpal, A., Smith, M. H. & Donlin, L. T. Rheumatology in the era of precision medicine: synovial tissue molecular patterns and treatment response in rheumatoid arthritis. Curr. Opin. Rheumatol. 33, 58–63 (2021).
van Baarsen, L. G. M. et al. Synovial tissue heterogeneity in rheumatoid arthritis in relation to disease activity and biomarkers in peripheral blood. Arthritis Rheumatol. 62, 1602–1607 (2010).
Dennis, G. et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res. Ther. 16, R90 (2014).
Orange, D. E. et al. Identification of three rheumatoid arthritis disease subtypes by machine learning integration of synovial histologic features and RNA sequencing data. Arthritis Rheumatol. 70, 690–701 (2018).
Lewis, M. J. et al. Molecular portraits of early rheumatoid arthritis identify clinical and treatment response phenotypes. Cell Rep. 28, 2455–2470 (2019).
Humby, F. et al. Synovial cellular and molecular signatures stratify clinical response to csDMARD therapy and predict radiographic progression in early rheumatoid arthritis patients. Ann. Rheumatol. Dis. 78, 761 (2019).
Nerviani, A. et al. A pauci-immune synovial pathotype predicts inadequate response to TNFα-blockade in rheumatoid arthritis patients. Front. Immunol. 11, 845 (2020).
Cohen, S. B. et al. Rituximab for rheumatoid arthritis refractory to anti–tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheumatol. 54, 2793–2806 (2006).
Humby, F. et al. Rituximab versus tocilizumab in anti-TNF inadequate responder patients with rheumatoid arthritis (R4RA): 16-week outcomes of a stratified, biopsy-driven, multicentre, open-label, phase 4 randomised controlled trial. Lancet 397, 305–317 (2021).
Cheng, L. et al. New insights from single-cell sequencing data: synovial fibroblasts and synovial macrophages in rheumatoid arthritis. Front. Immunol. 12, 709178 (2021).
Buckley, C. D., Ospelt, C., Gay, S. & Midwood, K. S. Location, location, location: how the tissue microenvironment affects inflammation in RA. Nat. Rev. Rheumatol. 17, 195–212 (2021).
Stephenson, W. et al. Single-cell RNA-seq of rheumatoid arthritis synovial tissue using low-cost microfluidic instrumentation. Nat. Commun. 9, 791 (2018).
Mizoguchi, F. et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 9, 789 (2018).
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Wei, K. et al. Notch signalling drives synovial fibroblast identity and arthritis pathology. Nature 582, 259–264 (2020).
Croft, A. P. et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 570, 246–251 (2019).
Orange, D. E. et al. RNA identification of PRIME cells predicting rheumatoid arthritis flares. N. Engl. J. Med. 383, 218–228 (2020).
Rao, D. A. et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114 (2017).
Ocampo, D. V. & Gladman, D. Psoriatic arthritis. F1000Res. 8, F1000 Faculty Rev-1665 (2019).
Diani, M., Altomare, G. & Reali, E. T helper cell subsets in clinical manifestations of psoriasis. J. Immunol. Res. 2016, 1–7 (2016).
Castillo, R. & Scher, J. U. Not your average joint: towards precision medicine in psoriatic arthritis. Clin. Immunol. 217, 108470 (2020).
Ritchlin, C. et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann. Rheumatol. Dis. 73, 990–999 (2014).
Mease, P. J. et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N. Engl. J. Med. 373, 1329–1339 (2015).
Belasco, J. et al. Comparative genomic profiling of synovium versus skin lesions in psoriatic arthritis. Arthritis Rheumatol. 67, 934–944 (2015).
Nerviani, A. et al. IL-23 skin and joint profiling in psoriatic arthritis: novel perspectives in understanding clinical responses to IL-23 inhibitors. Ann. Rheumatol. Dis. 80, 591–597 (2021).
Jadon, D. R., Stober, C., Pennington, S. R. & FitzGerald, O. Applying precision medicine to unmet clinical needs in psoriatic disease. Nat. Rev. Rheumatol. 16, 609–627 (2020).
Miyagawa, I. et al. Precision medicine using different biological DMARDs based on characteristic phenotypes of peripheral T helper cells in psoriatic arthritis. Rheumatol. 58, 336–344 (2018).
Rönnblom, L. & Leonard, D. Interferon pathway in SLE: one key to unlocking the mystery of the disease. Lupus Sci. Med. 6, e000270 (2019).
Morand, E. F. et al. Trial of anifrolumab in active systemic lupus erythematosus. N. Engl. J. Med. 382, 211–221 (2020).
Furie, R. et al. Anifrolumab, an anti–interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. 69, 376–386 (2017).
Furie, R. A. et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1) a randomised, controlled, phase 3 trial. Lancet Rheumatol. 1, E208–E219 (2019).
Baechler, E. C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl Acad. Sci. USA 100, 2610–2615 (2003).
Lyons, P. A. et al. Novel expression signatures identified by transcriptional analysis of separated leucocyte subsets in systemic lupus erythematosus and vasculitis. Ann. Rheumatol. Dis. 69, 1208 (2010).
Banchereau, R. et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell 165, 551–565 (2016).
Toro-Domínguez, D. et al. Stratification of systemic lupus erythematosus patients into three groups of disease activity progression according to longitudinal gene expression. Arthritis Rheumatol. 70, 2025–2035 (2018).
Panousis, N. I. et al. Combined genetic and transcriptome analysis of patients with SLE: distinct, targetable signatures for susceptibility and severity. Ann. Rheumatol. Dis. 78, 1079 (2019).
Figgett, W. A. et al. Machine learning applied to whole-blood RNA-sequencing data uncovers distinct subsets of patients with systemic lupus erythematosus. Clin. Transl. Immunol. 8, e01093 (2019).
Sandling, J. K. et al. Molecular pathways in patients with systemic lupus erythematosus revealed by gene-centred DNA sequencing. Ann. Rheumatol. Dis. 80, 109–117 (2021).
Jeffries, M. et al. Genome-wide DNA methylation patterns in CD4+ T cells from patients with systemic lupus erythematosus. Epigenetics 6, 593–601 (2011).
Renauer, P. et al. DNA methylation patterns in naïve CD4+ T cells identify epigenetic susceptibility loci for malar rash and discoid rash in systemic lupus erythematosus. Lupus Sci. Med. 2, e000101 (2015).
Coit, P. et al. Renal involvement in lupus is characterized by unique DNA methylation changes in naïve CD4+ T cells. J. Autoimmun. 61, 29–35 (2015).
Kessel, A. et al. Antibody clustering helps refine lupus prognosis. Semin. Arthritis Rheumatol. 39, 66–70 (2009).
Artim-Esen, B. et al. Cluster analysis of autoantibodies in 852 patients with systemic lupus erythematosus from a single center. J. Rheumatol. 41, 1304–1310 (2014).
Pacheco, Y. et al. Cytokine and autoantibody clusters interaction in systemic lupus erythematosus. J. Transl. Med. 15, 239 (2017).
Reynolds, J. A. et al. Cytokine profiling in active and quiescent SLE reveals distinct patient subpopulations. Arthritis Res. Ther. 20, 173 (2018).
Robinson, G. A. et al. Disease-associated and patient-specific immune cell signatures in juvenile-onset systemic lupus erythematosus: patient stratification using a machine-learning approach. Lancet Rheumatol. 2, e485–e496 (2020).
Toro-Domínguez, D. et al. Differential treatments based on drug-induced gene expression signatures and longitudinal systemic lupus erythematosus stratification. Sci. Rep. 9, 15502 (2019).
Guthridge, J. M. et al. Adults with systemic lupus exhibit distinct molecular phenotypes in a cross-sectional study. EClinicalMedicine 20, 100291 (2020).
Nehar-Belaid, D. et al. Mapping systemic lupus erythematosus heterogeneity at the single-cell level. Nat. Immunol. 21, 1094–1106 (2020).
Arazi, A. et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 20, 902–914 (2019).
Der, E. et al. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat. Immunol. 20, 915–927 (2019).
Fava, A. et al. Integrated urine proteomics and renal single-cell genomics identify an IFN-gamma response gradient in lupus nephritis. JCI Insight 5, e138345 (2020).
Fava, A. et al. Urine proteomics and renal single-cell transcriptomics implicate interleukin-16 in lupus nephritis. Arthritis Rheumatol. 74, 829–839 (2021).
Jennette, J. C. et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheumatol. 65, 1–11 (2013).
Suwanchote, S. et al. Anti-neutrophil cytoplasmic antibodies and their clinical significance. Clin. Rheumatol. 37, 875–884 (2018).
McKinney, E. F. et al. A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat. Med. 16, 586–591 (2010).
McKinney, E. F., Lee, J. C., Jayne, D. R. W., Lyons, P. A. & Smith, K. G. C. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 523, 612–616 (2015).
Lyons, P. A. et al. Genetically distinct subsets within ANCA-associated vasculitis. N. Engl. J. Med. 367, 214–223 (2012).
Lyons, P. A. et al. Genome-wide association study of eosinophilic granulomatosis with polyangiitis reveals genomic loci stratified by ANCA status. Nat. Commun. 10, 5120 (2019).
Stone, J. H. et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N. Engl. J. Med. 363, 221–232 (2010).
Unizony, S. et al. Clinical outcomes of treatment of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis based on ANCA type. Ann. Rheumatol. Dis. 75, 1166 (2016).
Jones, R. B. et al. Mycophenolate mofetil versus cyclophosphamide for remission induction in ANCA-associated vasculitis: a randomised, non-inferiority trial. Ann. Rheumatol. Dis. 78, 399 (2019).
Allanore, Y. et al. Systemic sclerosis. Nat. Rev. Dis. Prim. 1, 15002 (2015).
LeRoy, E. C. et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J. Rheumatol. 15, 202–205 (1988).
Johnson, S. R., Feldman, B. M. & Hawker, G. A. Classification criteria for systemic sclerosis subsets. J. Rheumatol. 34, 1855–1863 (2007).
Yang, C., Tang, S., Zhu, D., Ding, Y. & Qiao, J. Classical disease-specific autoantibodies in systemic sclerosis: clinical features, gene susceptibility, and disease stratification. Front. Med. 7, 587773 (2020).
Kayser, C. & Fritzler, M. J. Autoantibodies in systemic sclerosis: unanswered questions. Front. Immunol. 6, 167 (2015).
Leclair, V. et al. Subsets in systemic sclerosis: one size does not fit all. J. Scleroderma Relat. Disord. 1, 298–306 (2016).
Sobanski, V. et al. Phenotypes determined by cluster analysis and their survival in the prospective european scleroderma trials and research cohort of patients with systemic sclerosis. Arthritis Rheumatol. 71, 1553–1570 (2019).
Whitfield, M. L. et al. Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc. Natl Acad. Sci. USA 100, 12319–12324 (2003).
Gardner, H. et al. Gene profiling of scleroderma skin reveals robust signatures of disease that are imperfectly reflected in the transcript profiles of explanted fibroblasts. Arthritis Rheumatol. 54, 1961–1973 (2006).
Milano, A. et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS ONE 3, e2696 (2008).
Pendergrass, S. A. et al. Intrinsic gene expression subsets of diffuse cutaneous systemic sclerosis are stable in serial skin biopsies. J. Invest. Dermatol. 132, 1363–1373 (2012).
Hinchcliff, M. et al. Molecular signatures in skin associated with clinical improvement during mycophenolate treatment in systemic sclerosis. J. Invest. Dermatol. 133, 1979–1989 (2013).
Johnson, M. E. et al. Experimentally-derived fibroblast gene signatures identify molecular pathways associated with distinct subsets of systemic sclerosis patients in three independent cohorts. PLoS ONE 10, e0114017 (2015).
Taroni, J. N. et al. Molecular characterization of systemic sclerosis esophageal pathology identifies inflammatory and proliferative signatures. Arthritis Res. Ther. 17, 194 (2015).
Assassi, S. et al. Dissecting the heterogeneity of skin gene expression patterns in systemic sclerosis. Arthritis Rheumatol. 67, 3016–3026 (2015).
Mahoney, J. M. et al. Systems level analysis of systemic sclerosis shows a network of immune and profibrotic pathways connected with genetic polymorphisms. PLoS Comput. Biol. 11, e1004005 (2015).
Taroni, J. N. et al. A novel multi-network approach reveals tissue-specific cellular modulators of fibrosis in systemic sclerosis. Genome Med. 9, 27 (2017).
Chakravarty, E. F. et al. Gene expression changes reflect clinical response in a placebo-controlled randomized trial of abatacept in patients with diffuse cutaneous systemic sclerosis. Arthritis Res. Ther. 17, 159 (2015).
Khanna, D. et al. Abatacept in early diffuse cutaneous systemic sclerosis: results of a phase II investigator-initiated, multicenter, double-blind, randomized, placebo-controlled trial. Arthritis Rheumatol. 72, 125–136 (2020).
Martyanov, V. et al. Novel lung imaging biomarkers and skin gene expression subsetting in dasatinib treatment of systemic sclerosis-associated interstitial lung disease. PLoS ONE 12, e0187580 (2017).
Chung, L. et al. Molecular framework for response to imatinib mesylate in systemic sclerosis. Arthritis Rheumatol. 60, 584–591 (2009).
Franks, J. M. et al. A machine learning classifier for assigning individual patients with systemic sclerosis to intrinsic molecular subsets. Arthritis Rheumatol. 71, 1701–1710 (2019).
Brito-Zerón, P. et al. Sjögren syndrome. Nat. Rev. Dis. Prim. 2, 16047 (2016).
Hjelmervik, T. O. R., Petersen, K., Jonassen, I., Jonsson, R. & Bolstad, A. I. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjögren’s syndrome patients from healthy control subjects. Arthritis Rheumatol. 52, 1534–1544 (2005).
Gottenberg, J.-E. et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren’s syndrome. Proc. Natl Acad. Sci. USA 103, 2770–2775 (2006).
Wildenberg, M. E., Helden-Meeuwsen, C. G., van de Merwe, J. P., Drexhage, H. A. & Versnel, M. A. Systemic increase in type I interferon activity in Sjögren’s syndrome: a putative role for plasmacytoid dendritic cells. Eur. J. Immunol. 38, 2024–2033 (2008).
Emamian, E. S. et al. Peripheral blood gene expression profiling in Sjögren’s syndrome. Genes Immun. 10, 285–296 (2009).
Hall, J. C. et al. Precise probes of type II interferon activity define the origin of interferon signatures in target tissues in rheumatic diseases. Proc. Natl Acad. Sci. USA 109, 17609–17614 (2012).
Nezos, A. et al. Type I and II interferon signatures in Sjogren’s syndrome pathogenesis: contributions in distinct clinical phenotypes and Sjogren’s related lymphomagenesis. J. Autoimmun. 63, 47–58 (2015).
Brkic, Z. et al. Prevalence of interferon type I signature in CD14 monocytes of patients with Sjögren’s syndrome and association with disease activity and BAFF gene expression. Ann. Rheumatol. Dis. 72, 728 (2013).
Bodewes, I. L. A. et al. Systemic interferon type I and type II signatures in primary Sjögren’s syndrome reveal differences in biological disease activity. Rheumatol. 57, 921–930 (2018).
Hall, J. C. et al. Molecular subsetting of interferon pathways in Sjögren’s syndrome. Arthritis Rheumatol. 67, 2437–2446 (2015).
James, J. A. et al. Unique Sjögren’s syndrome patient subsets defined by molecular features. Rheumatol. 59, 860–868 (2019).
Soret, P. et al. A new molecular classification to drive precision treatment strategies in primary Sjögren’s syndrome. Nat. Commun. 12, 3523 (2021).
Isenberg, D. A. et al. An assessment of disease flare in patients with systemic lupus erythematosus: a comparison of BILAG 2004 and the flare version of SELENA. Ann. Rheumatol. Dis. 70, 54–59 (2011).
Yazar, S. et al. Single-cell eQTL mapping identifies cell type-specific genetic control of autoimmune disease. Science 376, eabf3041 (2022).
Perez, R. K. et al. Single-cell RNA-seq reveals cell type-specific molecular and genetic associations to lupus. Science 376, eabf1970 (2022).
Barturen, G. et al. Integrative analysis reveals a molecular stratification of systemic autoimmune diseases. Arthritis Rheumatol. 73, 1073–1085 (2021).
Merrill, J. T. et al. The biomarkers of lupus disease study: a bold approach may mitigate interference of background immunosuppressants in clinical trials. Arthritis Rheumatol. 69, 1257–1266 (2017).
Catalina, M. D. et al. Patient ancestry significantly contributes to molecular heterogeneity of systemic lupus erythematosus. JCI Insight 5, e140380 (2020).
Lewis, M. J. & Jawad, A. S. The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematosus. Rheumatol. 56, i67–i77 (2017).
Ngo, S. T., Steyn, F. J. & McCombe, P. A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 35, 347–369 (2014).
Andreoletti, G. et al. Transcriptomic analysis of immune cells in a multi-ethnic cohort of systemic lupus erythematosus patients identifies ethnicity- and disease-specific expression signatures. Commun. Biol. 4, 488 (2021).
Kuo, D. et al. HBEGF+ macrophages in rheumatoid arthritis induce fibroblast invasiveness. Sci. Transl. Med. 11, eaau8587 (2019).
Bonventre, J. V. Kidney organoids-a new tool for kidney therapeutic development. Kidney Int. 94, 1040–1042 (2018).
Barturen, G., Beretta, L., Cervera, R., Vollenhoven, R. V. & Alarcón-Riquelme, M. E. Moving towards a molecular taxonomy of autoimmune rheumatic diseases. Nat. Rev. Rheumatol. 14, 75–93 (2018).
Kroef, M. et al. Cytometry by time of flight identifies distinct signatures in patients with systemic sclerosis, systemic lupus erythematosus and Sjögrens syndrome. Eur. J. Immunol. 50, 119–129 (2020).
Martin-Gutierrez, L. et al. Stratification of patients with Sjögren’s syndrome and patients with systemic lupus erythematosus according to two shared immune cell signatures, with potential therapeutic implications. Arthritis Rheumatol. 73, 1626–1637 (2021).
Slight-Webb, S. et al. Unique serum immune phenotypes stratify Oklahoma Native American rheumatic disease patients. Arthritis Care Res. https://doi.org/10.1002/acr.24795 (2021).
Simon, Q. et al. A proinflammatory cytokine network profile in TH1/type 1 effector B cells delineates a common group of patients in four systemic autoimmune diseases. Arthritis Rheumatol. 73, 1550–1561 (2021).
Sirota, M. et al. Discovery and preclinical validation of drug indications using compendia of public gene expression data. Sci. Transl. Med. 3, 96ra77 (2011).
Acknowledgements
We thank S. Slight-Webb for critical review of the manuscript. We would like to thank the scientists in the Accelerating Medicines Partnerships in RA/Lupus and in Autoimmune and Immune Mediated Disease Networks, along with the NIAID Autoimmunity Centers of Excellence Network. This work is supported by NIH grants P30AR073750, U54GM104938, UC2AR081032, UM2AR067678, and UM1AI144292.
Author information
Authors and Affiliations
Contributions
All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Janet Pope and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling editor: Karen O’Leary, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1
Rights and permissions
About this article
Cite this article
Guthridge, J.M., Wagner, C.A. & James, J.A. The promise of precision medicine in rheumatology. Nat Med 28, 1363–1371 (2022). https://doi.org/10.1038/s41591-022-01880-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-022-01880-6
This article is cited by
-
Bioinformatics analyses of combined databases identify shared differentially expressed genes in cancer and autoimmune disease
Journal of Translational Medicine (2023)
-
Stratified distribution of Th17 and Treg cells in patients with multi-stage rheumatoid arthritis
Arthritis Research & Therapy (2023)