Abstract
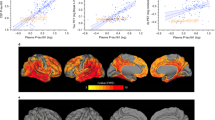
Plasma phosphorylated tau 181 (P-tau181) and 217 (P-tau217) are indicators of both amyloid and tau pathology in clinical settings, but their performance in heterogeneous community-based populations is unclear. We examined P-tau181 and P-tau217 (n = 1,329, aged 30–98 years), in the population-based Mayo Clinic Study of Aging. Continuous, unadjusted plasma P-tau181 and P-tau217 predicted abnormal amyloid positron-emission tomography (PET) (area under the receiver operating characteristic curve (AUROC) = 0.81–0.86) and tau PET entorhinal cortex (AUROC > 0.80), but was less predictive of a tau PET temporal region of interest (AUROC < 0.70). Multiple comorbidities were associated with higher plasma P-tau181 and P-tau217 levels; the difference between participants with and without chronic kidney disease (CKD) was similar to the difference between participants with and without elevated brain amyloid. The exclusion of participants with CKD and other comorbidities affected the establishment of a normal reference range and cutpoints. Understanding the effect of comorbidities on P-tau181 and P-tau217 levels is important for their future interpretation in the context of clinical screening, diagnosis or prognosis at the population level.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Raw and analyzed de-identified data from the MCSA can be requested at https://ras-rdrs.mayo.edu/Request/IndexRequest. The request will be reviewed by the MCSA investigators and Mayo Clinic to verify whether the request is subject to any intellectual property or confidentiality obligations. A data sharing agreement must be obtained prior to release.
Code availability
No custom code or mathematical algorithm that was central to the conclusions was used in this study.
Change history
10 October 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41591-022-02066-w
References
Mielke, M. M. et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 14, 989–997 (2018).
Janelidze, S. et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 26, 379–386 (2020).
Karikari, T. K. et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 19, 422–433 (2020).
Thijssen, E. H. et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 26, 387–397 (2020).
Palmqvist, S. et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 324, 772–781 (2020).
Mattsson-Carlgren, N. et al. Longitudinal plasma p-tau217 is increased in early stages of Alzheimer’s disease. Brain 143, 3234–3241 (2020).
Moscoso, A. et al. Time course of phosphorylated-tau181 in blood across the Alzheimer’s disease spectrum. Brain 144, 325–339 (2021).
Barthélemy, N. R., Horie, K., Sato, C. & Bateman, R. J. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J. Exp. Med. 217, e20200861 (2020).
Benussi, A. et al. Diagnostic and prognostic value of serum NfL and p-Tau181 in frontotemporal lobar degeneration. J. Neurol. Neurosurg. Psychiatry 91, 960–967 (2020).
Simrén, J. et al. The diagnostic and prognostic capabilities of plasma biomarkers in Alzheimer’s disease. Alzheimers Dement. 17, 1145–1156 (2021).
Mielke, M. M. et al. Comparison of plasma phosphorylated tau species with amyloid and tau positron emission tomography, neurodegeneration, vascular pathology, and cognitive outcomes. JAMA Neurol. 78, 1108–1117 (2021).
O’Bryant, S. E. et al. Blood-based biomarkers in Alzheimer disease: current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimers Dement. 13, 45–58 (2017).
Teunissen, C. E. et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol. 21, 66–77 (2022).
Mielke, M. M. Consideration of sex differences in the measurement and interpretation of Alzheimer disease-related biofluid-based biomarkers. J. Appl. Lab. Med. 5, 158–169 (2020).
Syrjanen, J. A. et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement. https://doi.org/10.1002/alz.12466 (online ahead of print).
Jack, C. R.Jr. et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement. 13, 205–216 (2017).
Lowe, V. J. et al. Tau-positron emission tomography correlates with neuropathology findings. Alzheimers Dement. 16, 561–571 (2020).
Ossenkoppele, R. et al. Tau PET correlates with different Alzheimer’s disease-related features compared to CSF and plasma p-tau biomarkers. EMBO Mol. Med. 13, e14398 (2021).
Brickman, A. M. et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 17, 1353–1364 (2021).
Ozarda, Y. Reference intervals: current status, recent developments and future considerations. Biochem. Med. (Zagreb) 26, 5–16 (2016).
Manrai, A. K., Patel, C. J. & Ioannidis, J. P. A. In the era of precision medicine and big data, who is normal? JAMA 319, 1981–1982 (2018).
Yoshihara, T. et al. Cerebrospinal fluid protein concentration in healthy older Japanese volunteers. Int. J. Environ. Res. Public Health 18, 8683 (2021).
Thijssen, E. H. et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol. 20, 739–752 (2021).
Roberts, R. O. et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 30, 58–69 (2008).
St Sauver, J. L. et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int. J. Epidemiol. 41, 1614–1624 (2012).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40, 373–383 (1987).
Beck, A. T., Steer, R. A. & Brown, G. K. Manual for the Beck Depression Inventory-II (Psychological Corporation, 1996).
Beck, A. T. & Steer, R. A. Beck Anxiety Inventory Manual (Psychological Corporation, 1993).
Ivnik, R. J. et al. Mayo’s Older Americans Normative Studies: updated AVLT norms for ages 56 to 97. Clin. Neuropsychol. 6, 83–104 (1992).
Petersen, R. C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256, 183–194 (2004).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (American Psychiatric Association, 1994).
Lowe, V. J. et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. J. Nucl. Med. 50, 878–886 (2009).
Price, J. C. et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J. Cereb. Blood Flow. Metab. 25, 1528–1547 (2005).
McNamee, R. L. et al. Consideration of optimal time window for Pittsburgh compound B PET summed uptake measurements. J. Nucl. Med. 50, 348–355 (2009).
Jack, C. R.Jr. et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain 131, 665–680 (2008).
Therneau, T. M. A package for survival analysis in R. R package version 3.2-7, https://CRAN.R-project.org/package=survival (2020).
Acknowledgements
Funding for this study was provided by grants from the National Institutes of Health (NIH) (U01 AG006786 to M.M.M., C.R.J. and R.C.P.; R37 AG011378 to C.R.J.; R01 NS097495 to P.V.; P30 AG062677 to R.C.P.; RF1 AG069052 to M.M.M., J.G-R. and P.V.; and R01 AG041851 to C.R.J. and D.S.K.) and the GHR Foundation (to R.C.P.). This study was made possible using the resources of the REP, which is supported by the NIH National Institute on Aging under award no. R01 AG034676. Meso Scale Discovery P-tau181 and P-tau217 assays were performed at Eli Lilly and Company. The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all data in the study, and all authors had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
M.M.M. and J.L.D. designed and planned this study. M.M.M. and R.D.F. designed the specific analysis. M.M.M. drafted the manuscript. All authors contributed to interpretation of the study results and revised and approved the manuscript for intellectual content. M.M.M. is the guarantor and accepts full responsibility for the work and conduct of the study, had access to the data and controlled the decision to publish. The corresponding author (M.M.M.) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Competing interests
M.M.M. has served as a consultant for Biogen, Brain Protection Company and Labcorp. J.L.D. was previously an employee and is still a minor shareholder of Eli Lilly and Company. J.L.D. is an inventor on patents or patent applications of Eli Lilly and Company relating to the assays, methods, reagents and/or compositions of matter used in this work. J.L.D. has also served as a consultant for Karuna Therapeutics and received research support from ADx Neurosciences, Roche Diagnostics and Eli Lilly and Company. R.D.F. and A.A.-S. report no conflicts. D.S.K. serves on a data safety monitoring board for the Dominantly Inherited Alzheimer Network (DIAN) study. D.S.K. also serves on a data safety monitoring board for Biogen but receives no personal compensation. D.S.K. is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals and the University of Southern California. D.S.K. serves as a consultant for Roche, Samus Therapeutics, Third Rock and Alzeca Biosciences but receives no personal compensation. V.J.L. received consulting fees from Bayer Schering Pharma, Piramal Life Sciences and Merck Research, and grants from GE Healthcare, Siemens Molecular Imaging, Avid Radiopharmaceuticals and the NIH. G.B. serves as a consultant for AbbVie, E-Scape and SciNeuro. P.V. received speaking fees from Miller Medical Communications. J.G.-R. receives NIH funding and serves as an assistant editor for Neurology. C.R.J. serves on an independent data monitoring board for Roche, has served as a speaker for Eisai and consulted for Biogen but receives no personal compensation from any commercial entity. C.R.J. receives research support from the NIH and the Alexander Family Alzheimer’s Disease Research Professorship of Mayo Clinic. R.C.P. received consulting fees from Hoffman-La Roche, Merck, Genentech, Biogen, GE Healthcare and Eisai.
Peer review
Peer review information
Nature Medicine thanks Julien Dumurgier, Suzanne Schindler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Jerome Staal, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Plasma P-tau181 and P-tau217 levels by clinical diagnosis and amyloid PET status.
Abnormal amyloid PET (A + ) was defined as standard uptake value ratio (SUVR) > 1.48 using PiB-PET. P-values are from two-sided Wilcoxon rank-sum tests without adjustment for multiple comparisons. Box plots display the median, 25th percentile, 75th percentile (middle, bottom, and top bars of the box), and the whiskers go out to 1.5 times the interquartile range (75th percentile – 25th percentile) from the 25th and 75th percentile. CU, cognitively unimpaired; MCI, mild cognitive impairment; DEM, dementia.
Extended Data Fig. 2 Plasma P-tau181 and P-tau217 levels by clinical diagnosis and amyloid and tau PET status.
Abnormal amyloid PET (A + ) was defined as standard uptake value ratio (SUVR) ≥ 1.48 using PiB-PET. Abnormal tau PET (T + ) meta region of interest (ROI) was defined as standard uptake value ratio (SUVR) ≥ 1.29 using AV1451, and included the amygdala, entorhinal cortex, fusiform, parahippocampal, and inferior temporal and middle temporal gyri. P-values are from two-sided Kruskal-Wallis tests. Box plots display the median, 25th percentile, 75th percentile (middle, bottom, and top bars of the box), and the whiskers go out to 1.5 times the interquartile range (75th percentile – 25th percentile) from the 25th and 75th percentile. CU, cognitively unimpaired; MCI, mild cognitive impairment.
Extended Data Fig. 3 Plasma P-tau181 and P-tau217 levels by presence of an APOE ɛ4 allele.
P-values are from two-sided Wilcoxon rank-sum tests without adjustment for multiple comparisons. Box plots display the median, 25th percentile, 75th percentile (middle, bottom, and top bars of the box), and the whiskers go out to 1.5 times the interquartile range (75th percentile – 25th percentile) from the 25th and 75th percentile. CU, cognitively unimpaired; MCI, mild cognitive impairment; DEM, dementia.
Extended Data Fig. 4 Plasma P-tau181 and P-tau217 levels by sex.
P-values are from two-sided Wilcoxon rank-sum tests without adjustment for multiple comparisons. Box plots display the median, 25th percentile, 75th percentile (middle, bottom, and top bars of the box), and the whiskers go out to 1.5 times the interquartile range (75th percentile – 25th percentile) from the 25th and 75th percentile. CU, cognitively unimpaired; MCI, mild cognitive impairment; DEM, dementia.
Extended Data Fig. 5 Forest plots of associations between multiple factors and plasma P-tau181 and P-tau217 levels using linear regression after excluding all participants with chronic kidney disease (n = 1231).
Black lines indicate univariable associations and red lines indicate associations after adjustment for age and sex. Means and 95% confidence intervals are provided. A + , Elevated amyloid PET; AFib, atrial fibrillation; BAI total, Beck Anxiety Inventory; BDI dep, Beck Depression Inventory; BMI, body mass index; Chemo, chemotherapy for those with a cancer diagnosis; DM, diabetes mellitus; HTN, hypertension; MI, myocardial infarction.
Supplementary information
Supplementary Information
Supplementary Tables 1–7.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mielke, M.M., Dage, J.L., Frank, R.D. et al. Performance of plasma phosphorylated tau 181 and 217 in the community. Nat Med 28, 1398–1405 (2022). https://doi.org/10.1038/s41591-022-01822-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-022-01822-2
This article is cited by
-
The impact of kidney function on plasma neurofilament light and phospho-tau 181 in a community-based cohort: the Shanghai Aging Study
Alzheimer's Research & Therapy (2024)
-
Exploring the potential of fully automated LUMIPULSE G plasma assays for detecting Alzheimer’s disease pathology
Alzheimer's Research & Therapy (2024)
-
Blood-based CNS regionally and neuronally enriched extracellular vesicles carrying pTau217 for Alzheimer’s disease diagnosis and differential diagnosis
Acta Neuropathologica Communications (2024)
-
Disease staging of Alzheimer’s disease using a CSF-based biomarker model
Nature Aging (2024)
-
Assessment of immunoprecipitation with subsequent immunoassays for the blood-based diagnosis of Alzheimer’s disease
European Archives of Psychiatry and Clinical Neuroscience (2024)