Abstract
Tay-Sachs disease (TSD) is an inherited neurological disorder caused by deficiency of hexosaminidase A (HexA). Here, we describe an adeno-associated virus (AAV) gene therapy expanded-access trial in two patients with infantile TSD (IND 18225) with safety as the primary endpoint and no secondary endpoints. Patient TSD-001 was treated at 30 months with an equimolar mix of AAVrh8-HEXA and AAVrh8-HEXB administered intrathecally (i.t.), with 75% of the total dose (1 × 1014 vector genomes (vg)) in the cisterna magna and 25% at the thoracolumbar junction. Patient TSD-002 was treated at 7 months by combined bilateral thalamic (1.5 × 1012 vg per thalamus) and i.t. infusion (3.9 × 1013 vg). Both patients were immunosuppressed. Injection procedures were well tolerated, with no vector-related adverse events (AEs) to date. Cerebrospinal fluid (CSF) HexA activity increased from baseline and remained stable in both patients. TSD-002 showed disease stabilization by 3 months after injection with ongoing myelination, a temporary deviation from the natural history of infantile TSD, but disease progression was evident at 6 months after treatment. TSD-001 remains seizure-free at 5 years of age on the same anticonvulsant therapy as before therapy. TSD-002 developed anticonvulsant-responsive seizures at 2 years of age. This study provides early safety and proof-of-concept data in humans for treatment of patients with TSD by AAV gene therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Requests for data presented in this article should be addressed to the corresponding authors (miguel.esteves@umassmed.edu or terry.flotte@umassmed.edu) and will be reviewed individually. Requests will be answered within 30 days from the date they are received. The data available for sharing include the study protocol, raw data for ELISpot assays, GM2 ganglioside quantification in CSF, hexosaminidase activity levels in serum and CSF and MRI data. All shared data will be deidentified to protect patient privacy. A data transfer and use agreement may be required after evaluation by the UMass Chan Medical School Office of Technology Management. The MRI Atlas of Myelination (www.myelinationmriatlas.com) was the data source in 7- and 12-month-old healthy children shown in Extended Data Fig. 1. Source data are provided with this paper.
References
Bley, A. E. et al. Natural history of infantile G(M2) gangliosidosis. Pediatrics 128, e1233–e1241 (2011).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377, 1713–1722 (2017).
Muramatsu, S. et al. A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson’s disease. Mol. Ther. 18, 1731–1735 (2010).
Hwu, W. L. et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency. Sci. Transl. Med. 4, 134ra161 (2012).
Passini, M. A. et al. Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice. J. Virol. 77, 7034–7040 (2003).
Gray, S. J., Nagabhushan Kalburgi, S., McCown, T. J. & Jude Samulski, R. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 20, 450–459 (2013).
Samaranch, L. et al. Strong cortical and spinal cord transduction after AAV7 and AAV9 delivery into the cerebrospinal fluid of nonhuman primates. Hum. Gene Ther. 24, 526–532 (2013).
Hinderer, C. et al. Intrathecal gene therapy corrects CNS pathology in a feline model of mucopolysaccharidosis I. Mol. Ther. 22, 2018–2027 (2014).
Meyer, K. et al. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose-response study in mice and nonhuman primates. Mol. Ther. 23, 477–487 (2015).
Miyanohara, A. et al. Potent spinal parenchymal AAV9-mediated gene delivery by subpial injection in adult rats and pigs. Mol. Ther. Methods Clin. Dev. 3, 16046 (2016).
Cearley, C. N. et al. Expanded repertoire of AAV vector serotypes mediate unique patterns of transduction in mouse brain. Mol. Ther. 16, 1710–1718 (2008).
Salegio, E. A. et al. Axonal transport of adeno-associated viral vectors is serotype-dependent. Gene Ther. 20, 348–352 (2013).
Passini, M. A., Lee, E. B., Heuer, G. G. & Wolfe, J. H. Distribution of a lysosomal enzyme in the adult brain by axonal transport and by cells of the rostral migratory stream. J. Neurosci. 22, 6437–6446 (2002).
Kells, A. P. et al. Efficient gene therapy-based method for the delivery of therapeutics to primate cortex. Proc. Natl Acad. Sci. USA 106, 2407–2411 (2009).
Baek, R. C. et al. AAV-mediated gene delivery in adult GM1-gangliosidosis mice corrects lysosomal storage in CNS and improves survival. PLoS One 5, e13468 (2010).
Cearley, C. N. & Wolfe, J. H. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J. Neurosci. 27, 9928–9940 (2007).
Dodge, J. C. et al. Gene transfer of human acid sphingomyelinase corrects neuropathology and motor deficits in a mouse model of Niemann-Pick type A disease. Proc. Natl Acad. Sci. USA 102, 17822–17827 (2005).
Foust, K. D., Flotte, T. R., Reier, P. J. & Mandel, R. J. Recombinant adeno-associated virus-mediated global anterograde delivery of glial cell line-derived neurotrophic factor to the spinal cord: comparison of rubrospinal and corticospinal tracts in the rat. Hum. Gene Ther. 19, 71–82 (2008).
Cachon-Gonzalez, M. B. et al. Effective gene therapy in an authentic model of Tay-Sachs-related diseases. Proc. Natl Acad. Sci. USA 103, 10373–10378 (2006).
Cachon-Gonzalez, M. B. et al. Gene transfer corrects acute GM2 gangliosidosis–potential therapeutic contribution of perivascular enzyme flow. Mol. Ther. 20, 1489–1500 (2012).
Gray-Edwards, H. et al. AAV gene therapy in a sheep model of Tay-Sachs disease. Hum. Gene Ther. 29, 312–326 (2018).
Rockwell, H. E. AAV-mediated gene delivery in a feline model of Sandhoff disease corrects lysosomal storage in the central nervous system. ASN Neuro. 7, 1759091415569908 (2015).
Taghian, T. et al. A safe and reliable technique for CNS delivery of AAV vectors in the cisterna magna.Mol. Ther. 28, 411–421 (2020).
Schneck, L., Maisel, J. & Volk, B. W. The startle response and serum enzyme profile in early detection of Tay-Sachs’ disease. J. Pediatr. 65, 749–756 (1964).
Mueller, C. et al. SOD1 suppression with adeno-associated virus and microRNA in familial ALS. N. Engl. J. Med. 383, 151–158 (2020).
Singh, A. MRI Atlas of Normal Myelination www.myelinationmriatlas.com (2017).
Hermoye, L. et al. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage 29, 493–504 (2006).
Mahuran, D. J. Biochemical consequences of mutations causing the GM2 gangliosidoses. Biochim. Biophys. Acta 1455, 105–138 (1999).
Neudorfer, O. et al. Late-onset Tay-Sachs disease: phenotypic characterization and genotypic correlations in 21 affected patients. Genet. Med. 7, 119–123 (2005).
Wendeler, M. & Sandhoff, K. Hexosaminidase assays. Glycoconj. J. 26, 945–952 (2009).
Baek, R. C., Martin, D. R., Cox, N. R. & Seyfried, T. N. Comparative analysis of brain lipids in mice, cats, and humans with Sandhoff disease. Lipids 44, 197–205 (2009).
Hepbildikler, S. T., Sandhoff, R., Kolzer, M., Proia, R. L. & Sandhoff, K. Physiological substrates for human lysosomal beta-hexosaminidase S. J. Biol. Chem. 277, 2562–2572 (2002).
Golebiowski, D. et al. Direct intracranial injection of AAVrh8 encoding monkey beta-N-acetylhexosaminidase causes neurotoxicity in the primate brain. Hum. Gene Ther. 28, 510–522 (2017).
Bradbury, A. M. et al. Therapeutic response in feline sandhoff disease despite immunity to intracranial gene therapy. Mol. Ther. 21, 1306–1315 (2013).
McCurdy, V. J. et al. Widespread correction of central nervous system disease after intracranial gene therapy in a feline model of Sandhoff disease. Gene Ther. 22, 181–189 (2015).
Gray-Edwards, H. L. et al. Adeno-associated virus gene therapy in a sheep model of Tay-Sachs disease. Hum. Gene Ther. 29, 312–326 (2018).
Taghian, T. et al. A safe and reliable technique for CNS delivery of AAV vectors in the cisterna magna. Mol. Ther. 28, 411–421 (2020).
Snyder, P. D. Jr., Krivit, W. & Sweeley, C. C. Generalized accumulation of neutral glycosphingolipids with G M2 ganglioside accumulation in the brain. J. Lipid Res. 13, 128–136 (1972).
Abe, T., Ogawa, K., Fuziwara, H., Urayama, K. & Nagashima, K. Spinal ganglia and peripheral nerves from a patent with Tay-Sachs disease. Morphological and ganglioside studies. Acta Neuropathol. 66, 239–244 (1985).
Rapin, I., Suzuki, K. & Valsamis, M. P. Adult (chronic) GM2 gangliosidosis: atypical spinocerebellar degeneration in a Jewish sibship. Arch. Neurol. 33, 120–130 (1976).
Tutunji, R. et al. Thalamic volume and dimensions on MRI in the pediatric population: normative values and correlations: a cross-sectional study. Eur. J. Radiol. 109, 27–32 (2018).
Taghian, T. et al. Volume and infusion rate dynamics of intraparenchymal central nervous system infusion in a large animal model. Hum. Gene Ther. 31, 617–625 (2020).
Corti, M. et al. B-cell depletion is protective against anti-AAV capsid immune response: a human subject case study. Mol. Ther. Methods Clin. Dev. 1, 14033 (2014).
Miyazaki, J. et al. Expression vector system based on the chicken beta-actin promoter directs efficient production of interleukin-5. Gene 79, 269–277 (1989).
Acknowledgements
This work was supported in part by the BlueGenes Foundation, which had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We are also grateful for the exceptional support from the National Tay-Sachs and Allied Diseases Association and the Cure Tay-Sachs Foundation during the preclinical development of this AAV gene therapy. We thank the families that provided MRI images from their deceased children with TSD or SD to support this study. We thank A. Singh for allowing us to use the MRI images from healthy children at 7 and 12 months of age for comparison purposes. We thank A. Zimmerman and B. Wong for their assistance during this study. We also thank N. Sanil and C. Dooley in the research pharmacy for their assistance in preparing the AAV doses for administration. We acknowledge the NEALS Biorepository for providing the biofluids from patient with amyotrophic lateral sclerosis used as non-TSD control samples in this study. M. Sena-Esteves was the sponsor of the clinical trial and was responsible for production of the AAVrh8 vectors in his laboratory as described in Methods. The hexosaminidase assays and western blots were also done in his laboratory. He had no role in the clinical aspects of the trial. During the manuscript review process, R.F. passed away in August 2021.
Author information
Authors and Affiliations
Contributions
T.R.F., R.H.B., O.C., R.M., A.P., S.B., S.G.S., J.P., A.A., L.G., C.J.T., R.F. and F.S.E. were part of the medical team. D.M.-Y., C.D. and A.M.K. were part of the clinical trial coordination team, data collection and analysis. P.W.L.T. analyzed the NGS data for the plasmids used in vector production. A.R.B. and M.S.-E. produced the AAVrh8 vectors. G.W., M.B. and C.M. performed all immunological assays and respective data interpretation. A.R.B. performed all enzyme assays and western blots, as well as respective data analysis and interpretation. B.A.B. performed the statistical analyses. A.A., S.R., Z.V., M.S.S. and M.G. were responsible for analysis and interpretation of MRI data. T.R.F., R.H.B., D.R.M., H.L.G.-E. and M.S.-E. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The University of Massachusetts Chan Medical School licensed the AAV vectors to Axovant Gene Therapies (now Sio Gene Therapies) in December 2018. Licensing revenue is shared with Auburn University and M.S.-E., D.R.M. and H.G.-E. receive part of the licensing revenue according to institutional policies. The schedule of licensing payments is governed by accomplishment of milestones related to an ongoing phase 1/2 clinical trial where the investigators named above have no role. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Timothy Yu, Steven Gray and Guilherme Baldo for their contribution to the peer review of this work. Anna Maria Ranzoni was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
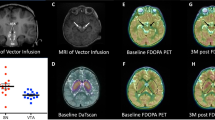
Extended Data Fig. 1 Structural brain MRI in healthy children during development and an infantile TSD patient.
T2 brain MRIs at 7 and 12 months of age in healthy children are from the MRI Atlas of Normal Myelination (www.myelinationmriatlas.com) with permission from Dr. Achint Singh. The T2 brain MRI of an 11-month-old infantile Tay-Sachs disease child was kindly provided by a family of a deceased child through the National Tay-Sachs and Allied Disease Association with permission to publish it here.
Extended Data Fig. 2 Volumetric calculations of lateral ventricular size and size of the lentiform nucleus.
TSD-001 has larger ventricles consistent with cortical atrophy whereas the relevance of TSD-002 ventricular size remains unclear and could be associated with normal brain development.
Extended Data Fig. 3 DTI of normal brain development.
Tractography of the normal developing brain from Hermoye et al, 2006. The color scheme of the tractography pathways is based on a standard red-green-blue (RGB) color code that shows the spatial locations of terminal regions of each pathway (right-left: red; dorsal-ventral: blue; and, anterior-posterior: green.
Extended Data Fig. 4 DTI results.
a, Mean diffusivity (MD) maps of TSD-002 brain indicating regions of interest (ROI) for calculation of DTI parameters. MD quantifications over time are displayed below the images for various ROIs in the brain for both TSD-001 and TSD-002. b, Axial diffusivity (AD) and c, radial diffusivity (RD) measurements are also shown for the same brain ROIs over time for both patients. Data is represented as mean ± SD of pixel intensity in the ROI. In TSD-002 both optic radiations show stable FA values and increased average diffusivity values. AD values remain stable even when FA decreases, as shown in the anterior corpus callosum for TSD-001.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1 and 2, Redacted certificates of analysis of AAV vector lots used in clinical trial
Source data
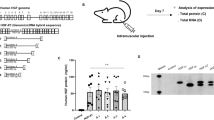
Source Data Fig. 1
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Flotte, T.R., Cataltepe, O., Puri, A. et al. AAV gene therapy for Tay-Sachs disease. Nat Med 28, 251–259 (2022). https://doi.org/10.1038/s41591-021-01664-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-021-01664-4
This article is cited by
-
Adeno-associated virus as a delivery vector for gene therapy of human diseases
Signal Transduction and Targeted Therapy (2024)
-
Acoustically targeted noninvasive gene therapy in large brain volumes
Gene Therapy (2024)
-
Biochemical and mutational analyses of HEXA in a cohort of Egyptian patients with infantile Tay-Sachs disease. Expansion of the mutation spectrum
Orphanet Journal of Rare Diseases (2023)
-
A year in review: brain barriers and brain fluids research in 2022
Fluids and Barriers of the CNS (2023)
-
AAV-based in vivo gene therapy for neurological disorders
Nature Reviews Drug Discovery (2023)