Abstract
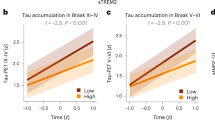
Compelling experimental evidence suggests that microglial activation is involved in the spread of tau tangles over the neocortex in Alzheimer’s disease (AD). We tested the hypothesis that the spatial propagation of microglial activation and tau accumulation colocalize in a Braak-like pattern in the living human brain. We studied 130 individuals across the aging and AD clinical spectrum with positron emission tomography brain imaging for microglial activation ([11C]PBR28), amyloid-β (Aβ) ([18F]AZD4694) and tau ([18F]MK-6240) pathologies. We further assessed microglial triggering receptor expressed on myeloid cells 2 (TREM2) cerebrospinal fluid (CSF) concentrations and brain gene expression patterns. We found that [11C]PBR28 correlated with CSF soluble TREM2 and showed regional distribution resembling TREM2 gene expression. Network analysis revealed that microglial activation and tau correlated hierarchically with each other following Braak-like stages. Regression analysis revealed that the longitudinal tau propagation pathways depended on the baseline microglia network rather than the tau network circuits. The co-occurrence of Aβ, tau and microglia abnormalities was the strongest predictor of cognitive impairment in our study population. Our findings support a model where an interaction between Aβ and activated microglia sets the pace for tau spread across Braak stages.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All requests for raw and analyzed data and materials will be promptly reviewed by McGill University to verify if the request is subject to any intellectual property or confidentiality obligations. Anonymized data will be shared upon request from a qualified academic investigator for the purpose of replicating the procedures and results presented in this article. Any data and materials that can be shared will be released via a material transfer agreement. Data are not publicly available due to information that could compromise the privacy of research participants.
Change history
15 October 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41591-021-01568-3
References
Ising, C. et al. NLRP3 inflammasome activation drives tau pathology. Nature 575, 669–673 (2019).
Salter, M. W. & Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 23, 1018–1027 (2017).
Sheffield, L. G., Marquis, J. G. & Berman, N. E. Regional distribution of cortical microglia parallels that of neurofibrillary tangles in Alzheimer’s disease. Neurosci. Lett. 285, 165–168 (2000).
Serrano-Pozo, A. et al. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am. J. Pathol. 179, 1373–1384 (2011).
Hopp, S. C. et al. The role of microglia in processing and spreading of bioactive tau seeds in Alzheimer’s disease. J. Neuroinflammation 15, 269 (2018).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991).
Braak, H. & Braak, E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol. Aging 18, 351–357 (1997).
Nelson, P. T. et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J. Neuropathol. Exp. Neurol. 71, 362–381 (2012).
Jack, C. R. Jr. et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 (2013).
Thal, D. R., Rüb, U., Orantes, M. & Braak, H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58, 1791–1800 (2002).
Dani, M. et al. Microglial activation correlates in vivo with both tau and amyloid in Alzheimer’s disease. Brain 141, 2740–2754 (2018).
Kreisl, W. C. et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain 136, 2228–2238 (2013).
Pascoal, T. A. et al. Aβ-induced vulnerability propagates via the brain’s default mode network. Nat. Commun. 10, 2353 (2019).
Owen, D. R. et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J. Cereb. Blood Flow Metab. 32, 1–5 (2012).
Bhatt, S. et al. PTSD is associated with neuroimmune suppression: evidence from PET imaging and postmortem transcriptomic studies. Nat. Commun. 11, 2360 (2020).
Kreisl, W. C. et al. PET imaging of neuroinflammation in neurological disorders. Lancet Neurol. 19, 940–950 (2020).
Pascoal, T. A. et al. In vivo quantification of neurofibrillary tangles with [18F]MK-6240. Alzheimers Res. Ther. 10, 74 (2018).
Pascoal, T. A. et al. 18F-MK-6240 PET for early and late detection of neurofibrillary tangles. Brain 143, 2818–2830 (2020).
Eikelenboom, P. et al. Neuroinflammation—an early event in both the history and pathogenesis of Alzheimer’s disease. Neurodegener. Dis. 7, 38–41 (2010).
Gratuze, M., Leyns, C. E. G. & Holtzman, D. M. New insights into the role of TREM2 in Alzheimer’s disease. Mol. Neurodegener. 13, 66 (2018).
Kreisl, W. C. et al. 11C-PBR28 binding to translocator protein increases with progression of Alzheimer’s disease. Neurobiol. Aging 44, 53–61 (2016).
Zou, J. et al. Microglial activation, but not tau pathology, is independently associated with amyloid positivity and memory impairment. Neurobiol. Aging 85, 11–21 (2020).
Hawrylycz, M. J. et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489, 391–399 (2012).
Brendel, M. et al. Increase of TREM2 during aging of an Alzheimer’s disease mouse model is paralleled by microglial activation and amyloidosis. Front. Aging Neurosci. 9, 8 (2017).
Piccio, L. et al. Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol. 131, 925–933 (2016).
Heslegrave, A. et al. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer’s disease. Mol. Neurodegener. 11, 3 (2016).
Jiang, T. et al. Silencing of TREM2 exacerbates tau pathology, neurodegenerative changes, and spatial learning deficits in P301S tau transgenic mice. Neurobiol. Aging 36, 3176–3186 (2015).
Cantarella, G. et al. Neutralization of TNFSF10 ameliorates functional outcome in a murine model of Alzheimer’s disease. Brain 138, 203–216 (2015).
Zhang, K. et al. CXCL1 contributes to β-amyloid-induced transendothelial migration of monocytes in Alzheimer’s disease. PLoS ONE 8, e72744 (2013).
Bolós, M. et al. Absence of CX3CR1 impairs the internalization of Tau by microglia. Mol. Neurodegener. 12, 59 (2017).
Ekert, J. O., Gould, R. L., Reynolds, G. & Howard, R. J. TNF alpha inhibitors in Alzheimer’s disease: a systematic review. Int. J. Geriatr. Psychiatry 33, 688–694 (2018).
Laurent, C., Buée, L. & Blum, D. Tau and neuroinflammation: what impact for Alzheimer’s disease and tauopathies? Biomed. J. 41, 21–33 (2018).
Faura, J. et al. CCL23: a chemokine associated with progression from mild cognitive impairment to Alzheimer’s disease. J. Alzheimers Dis. 73, 1585–1595 (2020).
McMahan, R. S. et al. Stromelysin-2 (MMP10) moderates inflammation by controlling macrophage activation. J. Immunol. 197, 899–909 (2016).
Zhang, Y., Anoopkumar-Dukie, S. & Davey, A. K. SIRT1 and SIRT2 modulators: potential anti-inflammatory treatment for depression? Biomolecules 11, 353 (2021).
Wang, X.-X., Tan, M.-S., Yu, J.-T. & Tan, L. Matrix metalloproteinases and their multiple roles in Alzheimer’s disease. Biomed Res. Int. 2014, 908636 (2014).
Willette, A. A. et al. Interleukin-8 and interleukin-10, brain volume and microstructure, and the influence of calorie restriction in old rhesus macaques. Age (Dordr.) 35, 2215–2227 (2013).
Rodriguez, J. A. et al. Metalloproteinases and atherothrombosis: MMP-10 mediates vascular remodeling promoted by inflammatory stimuli. Front. Biosci. 13, 2916–2921 (2008).
Braak, H., Thal, D. R., Ghebremedhin, E. & Del Tredici, K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 70, 960–969 (2011).
Kettenmann, H., Hanisch, U.-K., Noda, M. & Verkhratsky, A. Physiology of microglia. Physiol. Rev. 91, 461–553 (2011).
DeVos, S. L. et al. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci. Transl. Med. 9, eaag0481 (2017).
Takeda, S. et al. Seed-competent high-molecular-weight tau species accumulates in the cerebrospinal fluid of Alzheimer’s disease mouse model and human patients. Ann. Neurol. 80, 355–367 (2016).
Saman, S. et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 287, 3842–3849 (2012).
Fiandaca, M. S. et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement. 11, 600–607.e1 (2015).
Walsh, D. M. & Selkoe, D. J. A critical appraisal of the pathogenic protein spread hypothesis of neurodegeneration. Nat. Rev. Neurosci. 17, 251–260 (2016).
Sanchez, J. S. et al. The cortical origin and initial spread of medial temporal tauopathy in Alzheimer’s disease assessed with positron emission tomography. Sci. Transl. Med. 13, eabc0655 (2021).
Perez-Nievas, B. G. et al. Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain 136, 2510–2526 (2013).
Jack, C. R. Jr. et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 87, 539–547 (2016).
Grubman, A. et al. A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat. Neurosci. 22, 2087–2097 (2019).
Criscuolo, C. et al. Entorhinal cortex dysfunction can be rescued by inhibition of microglial RAGE in an Alzheimer’s disease mouse model. Sci. Rep. 7, 42370 (2017).
Edison, P. et al. Microglia, amyloid, and cognition in Alzheimer’s disease: an [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol. Dis. 32, 412–419 (2008).
Krasemann, S. et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581.e.9 (2017).
Hansen, D. V., Hanson, J. E. & Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 217, 459–472 (2018).
Lyoo, C. H. et al. Cerebellum can serve as a pseudo-reference region in Alzheimer disease to detect neuroinflammation measured with PET radioligand binding to translocator protein. J. Nucl. Med. 56, 701–706 (2015).
Liu, D. et al. Soluble TREM2 changes during the clinical course of Alzheimer’s disease: a meta-analysis. Neurosci. Lett. 686, 10–16 (2018).
Diedrichsen, J., Balsters, J. H., Flavell, J., Cussans, E. & Ramnani, N. A probabilistic MR atlas of the human cerebellum. Neuroimage 46, 39–46 (2009).
Therriault, J. et al. Determining amyloid-β positivity using 18F-AZD4694 PET imaging. J. Nucl. Med. 62, 247–252 (2021).
Pascoal, T. A. et al. Amyloid and tau signatures of brain metabolic decline in preclinical Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 45, 1021–1030 (2018).
Klein, A. & Tourville, J. 101 labeled brain images and a consistent human cortical labeling protocol. Front. Neurosci. 6, 171 (2012).
Gryglewski, G. et al. Spatial analysis and high resolution mapping of the human whole-brain transcriptome for integrative analysis in neuroimaging. Neuroimage 176, 259–267 (2018).
Jensen, C. S. et al. Exercise as a potential modulator of inflammation in patients with Alzheimer’s disease measured in cerebrospinal fluid and plasma. Exp. Gerontol. 121, 91–98 (2019).
Karikari, T. K. et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 19, 422–433 (2020).
Mathotaarachchi, S. et al. VoxelStats: a MATLAB package for multi-modal voxel-wise brain image analysis. Front. Neuroinform. 10, 20 (2016).
Acknowledgements
This research was supported by the Weston Brain Institute, Canadian Institutes of Health Research (CIHR) (no. MOP-11-51-31 and no. FRN, 152985 (P.R.-N.)), the Alzheimer’s Association (no. NIRG-12-92090 and no. NIRP-12-259245 (P.R.-N.)) and Fonds de Recherche du Québec–Santé (FRQS; Chercheur Boursier, no. 2020-VICO-279314 (P.R.-N.)). T.A.P., S.G. and P.R.-N. are members of the CIHR–Canadian Consortium of Neurodegeneration in Aging (CCNA), Canada Foundation for Innovation, CFI Project 34874. T.A.P. is supported by the Alzheimer’s Association (no. AACSF-20-648075). K.B. is supported by the Swedish Research Council (no. 2017-00915), the Alzheimer’s Drug Discovery Foundation (ADDF) (no. RDAPB-201809-2016615), the Swedish Alzheimer’s Foundation (no. AF-742881), Hjärnfonden (no. FO2017-0243), the Swedish State under the agreement between the Swedish government and the County Councils, ALF agreement (no. ALFGBG-715986) and European Union Joint Program for Neurodegenerative Disorders (no. JPND2019-466-236). H.Z. is a Wallenberg Scholar supported by grants from the Swedish Research Council (no. 2018-02532), European Research Council (no. 681712), Swedish State Support for Clinical Research (no. ALFGBG-720931), the ADDF (no. 201809-2016862) and UK Dementia Research Institute at University College London. The authors thank all study participants and staff of the McGill University Research Centre for Studies in Aging. We thank D. Jolly, A. Kostikov, M. Samoila-Lactatus, K. Ross, M. Boudjemeline and S. Li for assisting with the radiochemistry production. We also thank G. Gagne, C. Mayhew, T. Vinet-Celluci, K. Wan, S. Sbeiti, M. Jin Joung, M. Olmand, R. Nazar, H.-H. Hsiao, R. Bouhachi and A. Aliaga for consenting participants and/or helping with data acquisition. We thank Cerveau Technologies for the use of [18F]MK-6240.
Author information
Authors and Affiliations
Contributions
T.A.P., S.G. and P.R.-N. conceptualized the work. T.A.P., A.L.B., N.J.A. and P.R.-N. contributed to the design of the analyses. T.A.P., A.L.B., M.S.K., J.T., M.C., M. Savard, F.Z.L., C.T., T.K.K., J.O., S.M., J.S., G.M., J.-P.S., M.J.L., P.E. and P.R.-N. contributed to the acquisition, processing and analysis of the neuroimaging data. N.J.A., T.K.K., M. Schöll, K.B. and H.Z. contributed to the analysis of the fluid biomarkers. T.A.P. and P.R.-N. drafted the manuscript. All authors interpreted the data and contributed to revising the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Medicine thanks Michael Heneka and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Jerome Staal was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
Study flowchart.
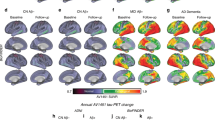
Extended Data Fig. 2 Cross-sectional and longitudinal abnormalities in [18F]MK-6240 across clinical groups.
(A) The brain images (cross-sectional analyses) show voxel-wise AUC results obtained from ROC curves between [18F]MK-6240 SUVR values of CU Aβ - versus CU Aβ + , MCI, or AD dementia. [18F]MK-6240 SUVR was increased in CU Aβ + , MCI, and AD dementia individuals in regions comprising early PET Braak-like stages, intermediary Braak stages, and across the whole brain cortex, respectively. Cross-sectional analysis was performed in 64 CU elderly (14 males, mean age = 72 (6)), 28 MCI (17 males, mean age = 73 (9)), and 16 AD dementia (6 males, mean age = 70 (8)). (B) The brain images (longitudinal analyses) show the results of voxel-wise paired t-test comparison between the baseline and follow-up [18F]MK-6240 SUVR images. CU, MCI, and AD dementia showed a more preeminent longitudinal [18F]MK-6240 SUVR increase in early, intermediary, and late Braak regions, respectively. Longitudinal analysis was performed in 34 CU elderly (6 males, mean age = 73 (6)), 13 MCI (9 males, mean age = 74 (6)), and 9 AD dementia (4 males, mean age = 70 (7)). Results survived to false discovery rate correction for multiple comparisons at P < 0.05.
Extended Data Fig. 3
Demographics and key characteristics of the population.
Extended Data Fig. 4 Groups difference in [11C]PBR28 SUVR.
(a) Averaged [11C]PBR28 SUVR maps, overlaid on a structural MRI template, suggest a progressively higher uptake in typical AD-related region in the posterior cingulate/precuneus, inferior parietal, and lateral temporal cortices from CU young (n = 22 (8 males, mean age = 23 (2)) to CU elderly (n = 64 (14 males, mean age = 72 (6)), MCI (n = 28, 17 males, mean age = 73 (9)), and AD dementia (n = 16, 6 males, mean age = 70 (8)) individuals. (b) Voxel-wise AUC maps obtained from ROC curves supported the above-mentioned differences between groups. Voxel-wise AUC also revealed the regions with higher [11C]PBR28 SUVR uptake in CU elderly Aβ + than CU elderly Aβ - and higher uptake in MCI Aβ + than MCI Aβ - (for example, medial temporal, posterior cingulate, and precuneus cortices). Young = cognitively unimpaired young; Elderly = cognitively unimpaired elderly; AD = AD dementia.
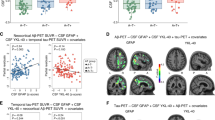
Extended Data Fig. 5 Microglial activation positively associates with brain Aβ and tau.
T-statistical parametric maps (false discovery rate corrected for multiple comparison at P < 0.05) overlaid on an MRI template show the results of voxel-wise linear regressions analysis between [11C]PBR28 SUVR and (A) Aβ [18F]AZD4694 SUVR and (B) tau [18F]MK-6240 SUVR. This analysis was performed in CU young (n = 22 (8 males, mean age = 23 (2)), CU elderly (n = 64 (14 males, mean age = 72 (6)), and 44 CI elderly (23 males, mean age = 72 (8)). The scatter plots show the results of two-side Pearson correlations between CSF sTREM2 and (C) CSF Aβ42/40 ratio and (D) CSF p-tau181 levels (CU young (n = 19 (9 males, mean age = 23 (2)), CU elderly (n = 29 (7 males, mean age = 73 (5)), and CI elderly (n = 27, 15 males, mean age = 71 (7)). The error bands denote 95% confidence intervals.
Extended Data Fig. 6 Microglia and tau networks correlations.
The plots show the correlations across PET Braak-like regions used in the network analyses presented in Fig. 2 for (a) [11C]PBR28 and (b) [18F]MK-6240. P values reflect the results of two-sided Pearson’s correlation between PET SUVR values corrected for age, sex, education, APOE ε4 status, and the remaining Braak regions not used in the given correlation. A correlation was interpreted as significant if it survived Bonferroni correction for multiple comparisons (30 tests, P < 0.0017). ** indicates a significant positive correlation; ## indicates a significant negative correlation. This analysis was performed in the elderly population (n = 108, 64 CU elderly (14 males, mean age = 72 (6)), 28 MCI (17 males, mean age = 73 (9)), and 16 AD dementia (6 males, mean age = 70 (8)).
Extended Data Fig. 7 Longitudinal tau propagation network correlations.
The plots show the correlations across PET Braak-like regions used in the longitudinal tau network analysis presented in Fig. 3A. P values reflect the results of two-sided Pearson’s correlation between changes in [18F]MK-6240 SUVR corrected for age, sex, education, APOE ε4 status, and the changes in the remaining Braak regions not used in the given correlation. A correlation was interpreted as significant if it survived Bonferroni correction for multiple comparisons (15 tests, P < 0.0034). ** indicates a significant positive correlation (n = 56, 34 CU (6 males, mean age = 73 (6)), 13 MCI (9 males, mean age = 74 (6)), and 9 AD dementia (4 males, mean age = 70 (7)).
Extended Data Fig. 8 Baseline microglial activation in Braak I region was associated with longitudinal tau accumulation over the neocortex.
The linear regression analysis shows that [11C]PBR28 SUVR value in the transentorhinal cortex (PET Braak-like stage I) was positively associated with 1-year change in tau PET uptake in brain regions comprising PET Braak-like stages II-VI, accounting for age, sex, APOE ε4 carriage status, and global Aβ load. The analysis was performed in 34 CU elderly (6 males, mean age = 73 (6)) and 22 CI elderly (13 males, mean age = 72 (7)). The error bands denote 95% confidence intervals.
Extended Data Fig. 9 The association between CSF sTREM2 and tau PET load recapitulates Braak stages.
The figure shows the results of voxel-wise regressions (false discovery rate corrected for multiple comparisons at P < 0.05) overlaid in a structure template (top) between CSF sTREM2 and [18F]MK-6240 SUVR in (A) CU (n = 48, 15 males, mean age = 53 (25)) and (B) MCI (n = 18, MCI (12 males, mean age = 72 (6)) participants. The bar plots (bottom) represent the percentage of the area showing a significant association between CSF sTREM2 and [18F]MK-6240 across Braak-like stage regions. The bars show that in CU individuals, CSF sTREM2 associates with tau pathology in early Braak stages, whereas in MCI individuals, CSF sTREM2 associates with tau pathology in late Braak stages, supporting a role for microglial activation in the spatial spread of tau in the human brain. No significant association was found in AD dementia patients after correction for multiple comparisons.
Extended Data Fig. 10 Distribution of Aβ, tau, and microglia activation abnormalities across diagnostic groups.
The figure shows the distribution of Aβ [18F]AZD4694, tau [18F]MK-6240, and microglia activation [11C]PBR28 abnormalities (+/-) in clinical groups. The co-occurrence of Aβ (A), tau (T), and microglia activation (MA) abnormalities (A/T/MA) was more prevalent in AD dementia (67%), followed by MCI (27%), CU elderly (4.5%), and CU young (0%). CU young (n = 22 (8 males, mean age = 23 (2)), CU elderly (n = 64 (14 males, mean age = 72 (6)), MCI (n = 28, 17 males, mean age = 73 (9)), and AD dementia (n = 16, 6 males, mean age = 70 (8)).
Supplementary information
Supplementary Information
Supplementary Figs. 1–4 and Tables 1–4.
Rights and permissions
About this article
Cite this article
Pascoal, T.A., Benedet, A.L., Ashton, N.J. et al. Microglial activation and tau propagate jointly across Braak stages. Nat Med 27, 1592–1599 (2021). https://doi.org/10.1038/s41591-021-01456-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-021-01456-w
This article is cited by
-
Onset of Alzheimer disease in apolipoprotein ɛ4 carriers is earlier in butyrylcholinesterase K variant carriers
BMC Neurology (2024)
-
Associations between cardiometabolic multimorbidity and cerebrospinal fluid biomarkers of Alzheimer’s disease pathology in cognitively intact adults: the CABLE study
Alzheimer's Research & Therapy (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
Microbial infection promotes amyloid pathology in a mouse model of Alzheimer’s disease via modulating γ-secretase
Molecular Psychiatry (2024)
-
Identification of senescent, TREM2-expressing microglia in aging and Alzheimer’s disease model mouse brain
Nature Neuroscience (2024)