Abstract
Agonism of the glucagon-like peptide-1 receptor (GLP-1R) results in glycemic lowering and body weight loss and is a therapeutic strategy to treat type 2 diabetes (T2D) and obesity. We developed danuglipron (PF-06882961), an oral small-molecule GLP-1R agonist and found it had comparable efficacy to injectable peptidic GLP-1R agonists in a humanized mouse model. We then completed a placebo-controlled, randomized, double-blind, multiple ascending-dose phase 1 study (NCT03538743), in which we enrolled 98 patients with T2D on background metformin and randomized them to receive multiple ascending doses of danuglipron or placebo for 28 d, across eight cohorts. The primary outcomes were assessment of adverse events (AEs), safety laboratory tests, vital signs and 12-lead electrocardiograms. Most AEs were mild, with nausea, dyspepsia and vomiting most commonly reported. There were no clinically meaningful AEs in laboratory values across groups. Heart rate generally increased with danuglipron treatment at day 28, but no heart-rate AEs were reported. Systolic blood pressure was slightly decreased and changes in diastolic blood pressure were similar with danuglipron treatment at day 28, compared with placebo. There were no clinically meaningful electrocardiogram findings. In this study in T2D, danuglipron was generally well tolerated, with a safety profile consistent with the mechanism of action of GLP-1R agonism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Upon request and subject to certain criteria, conditions and exceptions (https://www.pfizer.com/science/clinical-trials/trial-data-and-results), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. De-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer. Source data are provided with this paper.
References
International Diabetes Federation. IDF Diabetes Atlas 9th edn. (International Diabetes Federation, 2019).
Ali, M. K. et al. Achievement of goals in US diabetes care, 1999-2010. N. Engl. J. Med. 368, 1613–1624 (2013).
Kreymann, B., Williams, G., Ghatei, M. A. & Bloom, S. R. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 2, 1300–1304 (1987).
Nauck, M. A. et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am. J. Physiol. 273, E981–E988 (1997).
Flint, A., Raben, A., Astrup, A. & Holst, J. J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Invest. 101, 515–520 (1998).
Aroda, V. R. A review of GLP-1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes. Metab. 20, 22–33 (2018).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394, 121–130 (2019).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Drucker, D. J., Habener, J. F. & Holst, J. J. Discovery, characterization, and clinical development of the glucagon-like peptides. J. Clin. Invest. 127, 4217–4227 (2017).
Montvida, O., Shaw, J., Atherton, J. J., Stringer, F. & Paul, S. K. Long-term trends in antidiabetes drug usage in the US: real-world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care 41, 69–78 (2018).
Cooke, C. E., Lee, H. Y., Tong, Y. P. & Haines, S. T. Persistence with injectable antidiabetic agents in members with type 2 diabetes in a commercial managed care organization. Curr. Med. Res. Opin. 26, 231–238 (2010).
Holko, P., Kawalec, P. & Mossakowska, M. Quality of life related to oral, subcutaneous, and intravenous biologic treatment of inflammatory bowel disease: a time trade-off study. Eur. J. Gastroenterol. Hepatol. 30, 174–180 (2018).
Novo Nordisk A/S. RYBELSUS® (semaglutide) package insert (2020).
Buckley, S.T. et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aar7047 (2018).
Griffith, D.A. et al. A small-molecule oral agonist of the human glucagon-like peptide-1 receptor. Preprint at bioRxiv https://doi.org/10.1101/2020.09.29.319483 (2020).
Jun, L. S. et al. A novel humanized GLP-1 receptor model enables both affinity purification and Cre-LoxP deletion of the receptor. PLoS ONE 9, e93746 (2014).
Coskun, T. et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol. Metab. 18, 3–14 (2018).
Nauck, M. A. et al. A phase 2, randomized, dose-finding study of the novel once-weekly human GLP-1 analog, semaglutide, compared with placebo and open-label liraglutide in patients with type 2 diabetes. Diabetes Care 39, 231–241 (2016).
Drucker, D. J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 24, 15–30 (2016).
Lovshin, J. A. et al. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care 38, 132–139 (2015).
Liakos, A. et al. Effect of liraglutide on ambulatory blood pressure in patients with hypertension and type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Diabetes Obes. Metab. 21, 517–524 (2019).
Lastra, G., Syed, S., Kurukulasuriya, L. R., Manrique, C. & Sowers, J. R. Type 2 diabetes mellitus and hypertension: an update. Endocrinol. Metab. Clin. North Am. 43, 103–122 (2014).
Kjellsson, M. C., Cosson, V. F., Mazer, N. A., Frey, N. & Karlsson, M. O. A model-based approach to predict longitudinal HbA1c, using early phase glucose data from type 2 diabetes mellitus patients after anti-diabetic treatment. J. Clin. Pharmacol. 53, 589–600 (2013).
Lledó-García, R., Mazer, N. A. & Karlsson, M. O. A semi-mechanistic model of the relationship between average glucose and HbA1c in healthy and diabetic subjects. J. Pharmacokinet. Pharmacodyn. 40, 129–142 (2013).
Nathan, D. M. et al. Translating the A1C assay into estimated average glucose values. Diabetes Care 31, 1473–1478 (2008).
American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2020. Diabetes Care 43, S66–S76 (2020).
Deane, A. M. et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J. Clin. Endocrinol. Metab. 95, 215–221 (2010).
Lean, M. E. J. et al. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int J. Obes. 38, 689–697 (2014).
Fonseca, V.A. et al. Reductions in insulin resistance are mediated primarily via weight loss in subjects with type 2 diabetes on semaglutide. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/jc.2018-02685 (2019).
Shankar, S. S. et al. Standardized mixed-meal tolerance and arginine stimulation tests provide reproducible and complementary measures of β-cell function: results from the Foundation for the National Institutes of Health Biomarkers Consortium Investigative Series. Diabetes Care 39, 1602–1613 (2016).
Lin, P.-H. et al. Estimation of energy requirements in a controlled feeding trial. Am. J. Clin. Nutr. 77, 639–645 (2003).
Acknowledgements
This study was sponsored by Pfizer Inc. We thank the clinical study participants, the investigators and site coordinators. We thank J. Garren for providing support for nonclinical statistical analysis. Medical writing support, under the direction of the authors, was provided by E. Comeau, CMC Connect, McCann Health Medical Communications and was funded by Pfizer Inc. in accordance with Good Publication Practice guidelines.
Author information
Authors and Affiliations
Contributions
A.R.S. led the design, conduct and analysis of the clinical study, as well as the development of the manuscript. D.A.G. and R.M.E. led the design, conduct and analysis of the mouse studies. D.N.G. and C.B. contributed to the design, conduct and analysis of the clinical study. A.B. contributed to the analysis of the clinical study. A.M.K. contributed to the conceptualization and supervision of the clinical study presented, as well as the writing, review and editing of the manuscript. All authors critically reviewed the manuscript and approved the final draft for submission.
Corresponding author
Ethics declarations
Competing interests
All authors were employees of the sponsor at the time the study was conducted and are also Pfizer shareholders.
Additional information
Peer review information Nature Medicine thanks Michael Nauck, Stephen Bain, Victor Volovici and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Jennifer Sargent was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Liraglutide improved glucose tolerance and reduced food intake in wt and humanized GLP-1R mice.
(a, b) IPGTT study in wt and hGLP-1R mice after subcutaneous dosing with vehicle or liraglutide (0.3 mg/kg). Mice were dosed subcutaneously with vehicle or liraglutide 30 minutes prior to intraperitoneal administration of glucose (2 g/kg): (A) blood glucose; and (B) blood glucose AUC0-120. A total of 5 to 9 mice were used in each dose group. (C) Food intake in wt and hGLP-1R mice 15 hours after subcutaneous dosing with vehicle or liraglutide (0.3 mg/kg). A total of 12 to 17 mice were used in each dose group. Data are plotted as mean ± standard error. Plot (A) was analyzed using a mixed-effect model with AR(1) correlation and variance adjustments, and plots (B) and (C) were analyzed using one-way ANOVA, where **denotes p < 0.01, ***p < 0.001, and ****p < 0.0001. ANOVA, analysis of variance; AR(1), autoregressive model with lag=1; AUC0-120, area under the concentration–time curve from 0 to 120 minutes; hGLP-1R, humanized GLP-1R; IPGTT, intraperitoneal glucose tolerance test; Lira, liraglutide; ns, not significant; wt, wild type.
Extended Data Fig. 2 Danuglipron (PF-06882961) improved glucose tolerance and reduced food intake in an hGLP-1R mouse model.
(a–d), IPGTT study in wt and humanized hGLP-1R mice after subcutaneous dosing with vehicle or PF-06882961 (danuglipron) (3 mg/kg): (a) blood glucose; (b) blood glucose AUC0-120; (c) plasma insulin; and (d) plasma insulin AUC0-120. (e) Food intake in wt and hGLP-1R mice 2.5 hours (ZT = 13), 5.5 hours (ZT = 16), and 15.5 hours (ZT = 2) after subcutaneous dosing with vehicle or PF-06882961 (danuglipron) 30 mg/kg. A total of 6 to 7 mice were used in each dose group. Data are plotted as mean ± standard error. Plots (A), (B), and (E) were analyzed using a mixed-effect model with AR(1) correlation, and plots (C) and (D) were analyzed using two-way ANOVA, where *denotes P < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 (p values adjusted for multiple comparisons). (A) 0 min p = 0.0053; 15 min = <0.0001; 30 min p < 0.001; 45 min p < 0.0001; 60 min p < 0.0001; 90 min p < 0.0001; 120 min p = 0.0003. (B) ***p = 0.0001. (C) ***p = 0.0005, **p = 0.006. (D) *p = 0.032. (E) 2.5 hours p < 0.0001; 5.5 hours p < 0.0001; 15.5 hours p < 0.0001. ANOVA, analysis of variance; AR(1), autoregressive model with lag=1; AUC0-120, area under the concentration–time curve from 0 to 120 minutes; hGLP-1R, humanized GLP-1R; IPGTT, intraperitoneal glucose tolerance test; PF-‘2961, PF-06882961 (danuglipron); wt, wild type; ZT, zeitgeber time.
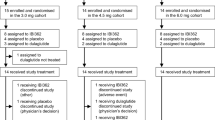
Extended Data Fig. 3 Study design.
*Represents danuglipron (PF-06882961) dose or matching placebo. 12 planned participants per cohort: 9 active and 3 placebo. 28 days of inpatient dosing for all cohorts. Titration occurred over the duration of the 28 days in the 120 mg BID ST, 200 mg QD CR, and 120 mg QD cohorts. BID, twice daily; CR, controlled-release; QD, once daily; ST, slow titration.
Extended Data Fig. 4 Time course of gastrointestinal TEAEs.
The plot presents the proportion of participants who experienced (A) diarrhea, (B) nausea, and (C) vomiting at Weeks 1, 2, 3, and 4. BID, twice daily; CR, controlled-release; QD, once daily; ST, slow titration; TEAE, treatment-emergent adverse event.
Extended Data Fig. 5 Absolute mean values in plasma glucose over 24 h on Day 28 by treatment arm.
The plot presents observed means for each treatment arm at each timepoint. BID, twice daily; CR, controlled-release; MDG, mean daily glucose; QD, once daily; ST, slow titration.
Extended Data Fig. 6 Effect of danuglipron (PF-06882961) on change from baseline in fasting plasma insulin and HOMA-IR at Day 28.
Danuglipron (PF-06882961) treatment was associated with a variable plasma insulin response, and HOMA-IR improved with the 70 mg BID and 120 mg BID doses. (a) CFB in FPI. (b) CFB in HOMA-IR. Plots represent LS means and 90% CIs from an MMRM model. Baseline was defined as the value collected at Day -1, 0 hours. HOMA-IR = (FPI x FPG)/405, where the FPI concentration is in μIU/mL and the FPG concentration is in mg/dL. (A) Placebo n = 25; 10 mg n = 9, p = 0.6115; 15 mg n = 8, p = 0.4340; 50 mg n = 8, p = 0.0080; 70 mg n = 9, p = 0.2777; 120 mg BID n = 9, p = 0.9292; 120 mg BID ST n = 9, p = 0.9663; 120 mg QD n = 8, p = 0.2792; 200 mg QD CR n = 7, p = 0.1075. (B) Placebo n = 25; 10 mg n = 9, p = 0.6947; 15 mg n = 8, p = 0.8518; 50 mg n = 8, p = 0.0287; 70 mg n = 9, p = 0.0787; 120 mg BID n = 9, p = 0.0788; 120 mg BID ST n = 9, p = 0.1089; 120 mg QD n = 8, p = 0.2535; 200 mg QD CR n = 7, p = 0.5326. *Statistical significance, compared with placebo, was pre-defined as a two-sided p value of <0.1 based on the t-statistic from the MMRM models. BID, twice daily; CFB, change from baseline; CI confidence interval; CR, controlled-release; FPI, fasting plasma insulin; HOMA-IR, homeostatic model assessment for insulin resistance; LS, least squares; QD, once daily; ST, slow titration.
Extended Data Fig. 7 Effect of danuglipron (PF-06882961) on mean body weight and HbA1c at Day 28.
a, b, Dosing with danuglipron (PF-06882961) resulted in dose-responsive CFB in body weight and HbA1c after 28 days of PF-06882961 administration. (a) CFB in body weight. (b) CFB in HbA1c. Data represent LS means and 90% CIs (a) based on an MMRM and (b) based on an ANCOVA model. Baseline was defined as the value collected at Day -1, 0 hours. (a) Placebo n = 25; 10 mg n = 9, p = 0.5769; 15 mg n = 8, p = 0.3949; 50 mg n = 8, p = 0.2491; 70 mg n = 9, p = 0.0023; 120 mg BID n = 9, p < 0.0001; 120 mg BID ST n = 9, p < 0.0001; 120 mg QD n = 8, p = 0.0008; 200 mg QD CR n = 7, p = 0.0542. (B) Placebo n = 25; 10 mg n = 9, p = 0.0167; 15 mg n = 8, p = 0.0237; 50 mg n = 8, p = 0.0004; 70 mg n = 9, p < 0.0001; 120 mg BID n = 9, p < 0.0001; 120 mg BID ST n = 9, p < 0.0001; 120 mg QD n = 8, p = 0.0010; 200 mg QD CR n = 7, p = 0.0006. *Statistical significance, compared with placebo, was pre-defined as a two-sided P value of <0.1 based on the t-statistic from the (a) MMRM model or (b) ANCOVA model. ANCOVA, analysis of covariance; BID, twice daily; CFB, change from baseline; CI, confidence interval; CR, controlled-release; HbA1c, glycated hemoglobin; LS, least squares; MMRM, mixed model repeated measures; QD, once daily; ST, slow titration.
Extended Data Fig. 8 Effect of danuglipron (PF-06882961) on change from baseline at Day 28 in AUC0-4 for glucose, insulin, glucagon, and C-peptide.
Danuglipron (PF-06882961) improved glucose AUC0-4 at all dose levels in a dose-responsive manner in 28 days, while insulin AUC0-4 response was variable, with statistically significant lowering at the 120 mg BID and 120 mg QD dose levels. (a) CFB in glucose AUC0-4. (b) CFB in insulin AUC0-4. (c) CFB in C-peptide AUC0-4. (d) CFB in glucagon AUC0-4. All plots show LS means and 90% CIs. Baseline was defined as the value collected at Day -1, 0 hours. (a) Placebo n = 25; 10 mg n = 9, p = 0.0234; 15 mg n = 8, p = 0.0034; 50 mg n = 8, p = 0.0320; 70 mg n = 9, p < 0.0001; 120 mg BID n = 9, p < 0.0001; 120 mg BID ST n = 9, p < 0.0001; 120 mg QD n = 8, p = 0.0004; 200 mg QD CR n = 7, p = 0.0002. (B) Placebo n = 24; 10 mg n = 8, p = 0.1674; 15 mg n = 8, p = 0.2985; 50 mg n = 8, p = 0.3293; 70 mg n = 8, p = 0.3562; 120 mg BID n = 9, p = 0.0030; 120 mg BID ST n = 9, p = 0.1025; 120 mg QD n = 8, p = 0.0074; 200 mg QD CR n = 7, p = 0.5884. (C) Placebo n = 25; 10 mg n = 9, p = 0.0056; 15 mg n = 8, p = 0.0479; 50 mg n = 8, p = 0.1520; 70 mg n = 8, p = 0.8454; 120 mg BID n = 9, p = 0.2467; 120 mg BID ST n = 9, p = 0.6303; 120 mg QD n = 8, p = 0.0255; 200 mg QD CR n = 7, p = 0.0856. (D) Placebo n = 25; 10 mg n = 9, p = 0.9681; 15 mg n = 8, p = 0.2274; 50 mg n = 8, p = 0.8698; 70 mg n = 8, p = 0.7941; 120 mg BID n = 9, p = 0.1005; 120 mg BID ST n = 9, p = 0.8800; 120 mg QD n = 8, p = 0.3025; 200 mg QD CR n = 7, p = 0.7519. *Statistical significance, compared with placebo, was pre-defined as a two-sided p value of <0.1 based on the t-statistic from the (a, b) MMRM models or (c, d) ANCOVA models. AUC0-4, area under the concentration–time curve from 0 to 4 hours; BID, twice daily; CI, confidence interval; CR, controlled-release; LS, least squares; QD, once daily; ST, slow titration.
Extended Data Fig. 9 Danuglipron (PF-06882961) pharmacokinetic concentration–time profiles at Day 28 (semi-log scale).
Danuglipron (PF-06882961) exposures increased in an approximately dose-proportional manner across BID and QD dosing regimens. The LLOQ was 0.100 ng/mL. Summary statistics were calculated by setting concentration values below the LLOQ to zero. If a participant received a dose that was not assigned based on the randomized titration scheme, the data from that day were not included. BID, twice daily; CR, controlled-release; LLOQ, lower limit of quantification; QD, once daily; ST, slow titration.
Supplementary information
Supplementary Information
Supplementary Tables 1–5.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
About this article
Cite this article
Saxena, A.R., Gorman, D.N., Esquejo, R.M. et al. Danuglipron (PF-06882961) in type 2 diabetes: a randomized, placebo-controlled, multiple ascending-dose phase 1 trial. Nat Med 27, 1079–1087 (2021). https://doi.org/10.1038/s41591-021-01391-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-021-01391-w
This article is cited by
-
Effect of Food Consumption on the Pharmacokinetics, Safety, and Tolerability of Once-Daily Orally Administered Orforglipron (LY3502970), a Non-peptide GLP-1 Receptor Agonist
Diabetes Therapy (2024)
-
SpaceGrow: efficient shape-based virtual screening of billion-sized combinatorial fragment spaces
Journal of Computer-Aided Molecular Design (2024)
-
GLP-1R signaling neighborhoods associate with the susceptibility to adverse drug reactions of incretin mimetics
Nature Communications (2023)
-
Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action
Nature Reviews Cardiology (2023)
-
Conserved class B GPCR activation by a biased intracellular agonist
Nature (2023)