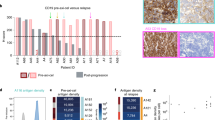
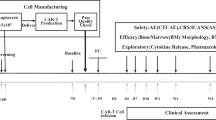
Abstract
Chimeric antigen receptor (CAR) T cells targeting CD19 are a breakthrough treatment for relapsed, refractory B cell malignancies1,2,3,4,5. Despite impressive outcomes, relapse with CD19− disease remains a challenge. We address this limitation through a first-in-human trial of bispecific anti-CD20, anti-CD19 (LV20.19) CAR T cells for relapsed, refractory B cell malignancies. Adult patients with B cell non-Hodgkin lymphoma or chronic lymphocytic leukemia were treated on a phase 1 dose escalation and expansion trial (NCT03019055) to evaluate the safety of 4-1BB–CD3ζ LV20.19 CAR T cells and the feasibility of on-site manufacturing using the CliniMACS Prodigy system. CAR T cell doses ranged from 2.5 × 105–2.5 × 106 cells per kg. Cell manufacturing was set at 14 d with the goal of infusing non-cryopreserved LV20.19 CAR T cells. The target dose of LV20.19 CAR T cells was met in all CAR-naive patients, and 22 patients received LV20.19 CAR T cells on protocol. In the absence of dose-limiting toxicity, a dose of 2.5 × 106 cells per kg was chosen for expansion. Grade 3–4 cytokine release syndrome occurred in one (5%) patient, and grade 3–4 neurotoxicity occurred in three (14%) patients. Eighteen (82%) patients achieved an overall response at day 28, 14 (64%) had a complete response, and 4 (18%) had a partial response. The overall response rate to the dose of 2.5 × 106 cells per kg with non-cryopreserved infusion (n = 12) was 100% (complete response, 92%; partial response, 8%). Notably, loss of the CD19 antigen was not seen in patients who relapsed or experienced treatment failure. In conclusion, on-site manufacturing and infusion of non-cryopreserved LV20.19 CAR T cells were feasible and therapeutically safe, showing low toxicity and high efficacy. Bispecific CARs may improve clinical responses by mitigating target antigen downregulation as a mechanism of relapse.
This is a preview of subscription content, access via your institution
Access options



Similar content being viewed by others
Data availability
The LV20.19 sequence is available in Extended Data Fig. 1, and patient-level response data are provided in Supplementary Table 1 and Fig. 1f. All requests for raw and analyzed data and materials will be promptly reviewed by the Medical College of Wisconsin and Lentigen Technology to verify whether the request is subject to any intellectual property or confidentiality obligations. Patient-related data not included in the paper were generated as part of clinical trials and may be subject to patient confidentiality. Any data and materials that can be shared will be released via a material transfer agreement. All other data that support the findings of this study will be provided by the corresponding authors upon reasonable request when possible.
References
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2018).
Schuster, S. J. et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 377, 2545–2554 (2017).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Tran, E., Longo, D. L. & Urba, W. J. A milestone for CAR T cells. N. Engl. J. Med. 377, 2593–2596 (2017).
Locke, F. L. et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 20, 31–42 (2019).
Abramson, J. S. et al. Pivotal safety and efficacy results from transcend NHL 001, a multicenter phase 1 study of lisocabtagene maraleucel (liso-cel) in relapsed/refractory (R/R) large B cell lymphomas. Blood 134, 241 (2019).
Sotillo, E. et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 5, 1282–1295 (2015).
Orlando, E. J. et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat. Med. 24, 1504–1506 (2018).
Shah, N. N. & Fry, T. J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 16, 372–385 (2019).
Hamieh, M. et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 568, 112–116 (2019).
Spiegel, J. Y. et al. Outcomes in large B-cell lymphoma progressing after axicabtagene ciloleucel (axi-cel): results from the US Lymphoma CAR-T Consortium. J. Clin. Oncol. 37, 7517 (2019).
Neelapu, S. S. et al. CD19 loss with preservation of other B cell lineage features in patients with large B cell lymphoma who relapsed post-axi-cel. Blood 134, 203 (2019).
Shah, N. N., Maatman, T., Hari, P. & Johnson, B. Multi-targeted CAR T-cell therapies for B-cell malignancies. Front. Oncol. 9, 146 (2019).
Majzner, R. G. & Mackall, C. L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 8, 1219–1226 (2018).
Zah, E. et al. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol. Res. 4, 498–508 (2016).
Schneider, D. et al. A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J. Immunother. Cancer 5, 42 (2017).
Lee, D. W. et al. ASBMT consensus grading for cytokine release syndrome and neurological toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 25, 625–638 (2018).
Teachey, D. T. et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 6, 664–679 (2016).
Shimabukuro-Vornhagen, A. et al. Cytokine release syndrome. J. Immunother. Cancer 6, 56 (2018).
Chou, C. K. & Turtle, C. J. Insight into mechanisms associated with cytokine release syndrome and neurotoxicity after CD19 CAR T-cell immunotherapy. Bone Marrow Transplant. 54, 780–784 (2019).
Hay, K. A. et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T cell therapy. Blood 130, 2295–2306 (2017).
Zhu, F. et al. Closed-system manufacturing of CD19 and dual-targeted CD20/19 chimeric antigen receptor T cells using the CliniMACS Prodigy device at an academic medical center. Cytotherapy 20, 394–406 (2018).
Tong C. et al. Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B cell lymphoma. Blood https://doi.org/10.1182/blood.2020005278 (2020).
Hossain, N. et al. Phase I experience with a bi-specific CAR targeting CD19 and CD22 in adults with B-cell malignancies. Blood 132, 490 (2018).
Pan, J. et al. Sequential CD19-22 CAR T therapy induces sustained remission in children with r/r B-ALL. Blood 135, 387–391 (2020).
Jeurink, P. V. et al. T cell responses in fresh and cryopreserved peripheral blood mononuclear cells: kinetics of cell viability, cellular subsets, proliferation, and cytokine production. Cryobiology 57, 91–103 (2008).
Panch, S. R. et al. Effect of cryopreservation on autologous chimeric antigen receptor T cell characteristics. Mol. Ther. 27, 1275–1285 (2019).
Cheson, B. D. et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J. Clin. Oncol. 32, 3059–3068 (2014).
Lee, D. W. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195 (2014).
US Department of Health and Human Services. Common Terminology Criteria for Adverse Events version 5.0 (US Department of Health and Human Services, 2017).
Hallek, M. et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute–Working Group 1996 guidelines. Blood 111, 5446–5456 (2008).
Acknowledgements
We thank all study participants and the BMT & Cellular Therapy team from the Medical College of Wisconsin for their care of these patients and acknowledge the contributions of laboratory staff from BMT & Cellular Therapy, in particular H. Xu, K. Chaney and L. Luib, who were involved in LV20.19 CAR T cell manufacturing, coordinators K. Worzalla and J. Esselmann for administrative and logistical support and S. Heidtke for laboratory quality management oversight. Additionally, we would like to thank the MACC Fund and the Children’s Wisconsin Foundation for their support of the Cell Therapy Lab. Lentigen Technology, a Miltenyi Biotec company, provided the lentiviral (LV20.19) vector used in this study and partial funding support for data collection. The Drive for the Cure Foundation and the Froedtert & Medical College of Wisconsin Cancer Center provided additional partial funding. The primary data analysis was conducted by N.N.S., B.D.J. and A.S.
Author information
Authors and Affiliations
Contributions
N.N.S. was involved in development of the concept and trial design, data collection and assembly, statistical analysis, interpretation, manuscript writing and final approval of the manuscript. B.D.J. was involved in cell manufacturing, data analysis, manuscript writing and final approval of the manuscript. D.S. was involved in vector design, concept development, manuscript writing and final approval of the manuscript. F.Z. was involved in data collection, manuscript writing and final approval of the manuscript. A.S. was involved in statistical analysis, manuscript writing and final approval of the manuscript. C.A.K.-T. was involved in manuscript writing and final approval of the manuscript. W.K. was involved in manuscript writing and final approval of the manuscript. A.A.W. was involved in manuscript writing and final approval of the manuscript. M.J.K. was involved in manuscript writing and final approval of the manuscript. S.Y. was involved in data collection, manuscript writing and final approval of the manuscript. A.C. was involved in data collection, pathology review, manuscript writing and final approval of the manuscript. M.H. was involved in data collection, manuscript writing and final approval of the manuscript. T.S.F. was involved in data collection, manuscript writing and final approval of the manuscript. B.D. was involved in vector design, concept development, manuscript writing and final approval of the manuscript. R.O. was involved in vector design, concept development, manuscript writing and final approval of the manuscript. P.H. was involved in development of the concept and trial design, data collection and assembly, statistical analysis, interpretation, manuscript writing and final approval of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
N.N.S. reports honoraria, travel support and research funding from Miltenyi Biotec; honoraria from Incyte and Celgene; service on scientific advisory boards for Kite, Celgene, TG Therapeutics and Lily; and institutional research support for clinical trials from BMS and Miltenyi Biotec. D.S. reports employment with Lentigen, a Miltenyi Biotec company. B.D.J. reports travel support and research funding from Miltenyi Biotec. A.S. has no competing financial interest with regard to this submission. F.Z. has no competing financial interests with regard to this submission. C.A.K.-T. has no competing financial interest with regard to this submission. W.K. reports employment with Lentigen, a Miltenyi Biotec company. A.A.W. reports employment with Lentigen, a Miltenyi Biotec company. M.J.K. reports employment with Lentigen, a Miltenyi Biotec company. S.Y. has no competing financial interest with regard to this submission. A.C. has no competing financial interest with regard to this submission. M.H. reports research support funding from Takeda Pharmaceutical Company and Spectrum Pharmaceuticals; consultancy with Incyte Corporation, ADC Therapeutics, Celgene, Pharmacyclics, Omeros and Verastem; and speaker’s bureau with Sanofi Genzyme and AstraZeneca. T.S.F. reports research funding from Millennium, Kyowa, TG Therapeutics, Portola and Curis; consulting honoraria from Genentech, Adaptive Biotechnologies, AbbVie, Verastem, Kite and MorphoSys; and speaking honoraria from Genentech, Sanofi, Seattle Genetics, AstraZeneca, Celgene and Adaptive Biotechnologies. B.D. reports employment with Lentigen, a Miltenyi Biotec company. R.O. reports prior employment and an ongoing consulting relationship with and research support from Lentigen, a Miltenyi Biotec company, and consulting for Umoja Biopharma. P.H. reports honoraria and travel support from Miltenyi Biotec; research grants from BMS, Takeda, Janssen and Bluebird; and honoraria from BMS, Takeda, Amgen, Karyopharm, Janssen, Incyte, Sanofi and GSK.
Additional information
Peer review information Javier Carmona was the primary editor on this article, and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 LV20.19 construct and sequence.
Anti-CD19 and anti-CD20 CAR constructs were generated by linking the single chain fragment variable (scFv) region of monoclonal antibody FMC-63 (CD19) and Leu-16 (CD20) with a CD8 hinge and transmembrane domain to the intracellular 4–1BB (CD137) and CD3ζ signaling domains. The CD20 and CD19 scFv were linked sequentially by a flexible Gly-Ser Linker17.
Extended Data Fig. 2 Consort Diagram.
Consort Diagram describing patient population.
Extended Data Fig. 3 Manufacturing data from 22 LV20.19 CAR T-cell products generated on the CliniMACS Prodigy.
Detailed manufacturing data of LV20.19 CAR T-cells from CliniMACS Prodigy runs (n = 22 patients) NA = not available.
Extended Data Fig. 5 Grade 1–2 adverse events in > 10% of patients.
Grade 1–2 Adverse Events by dose level; graded per CTCAE Version 5.031.
Extended Data Fig. 6 CD4:CD8 ratios of CAR+ and CAR- T-cells in the peripheral blood following infusion.
CD4/CD8 ratios are depicted for each individual patient in the Phase I study out to 1-year post-infusion. The flow cytometry gating strategy shown in Supplementary Figure 8 was used to determine Protein L + (CAR CD3) and Protein L- (Non CAR CD3) CD4/CD8 ratios. The Day 28 clinical response status for each patient is shown at the top.
Extended Data Fig. 7 LVCAR20.19 CAR T-cells in the peripheral blood CD3 compartment of patients given fresh cells versus those treated with cryopreserved/thawed cells.
Peripheral blood mononuclear cells were isolated pre- (Day 0) and post-infusion at the designated times and assessed for numbers of LVCAR20.19 CAR + T-cells in the blood by flow cytometry after staining with Protein L. (a, b) Compares CAR T-cell persistence in the blood of patients given fresh infusions (n = 15) versus those infused with cryo/thawed cells (n = 7). (c) AUC comparisons of curves in ‘Panel a’ versus curves in ‘Panel b’ were determined using GraphPad Prism software. (d) Compares peak CAR + CD3 T-cell numbers from subjects in ‘Panel a’ versus subjects in ‘Panel b’. The dark horizontal lines in panels c and d represent median group values. The flow cytometry gating strategy shown in Supplementary Figure 8 was used to determine Protein L + (CAR + ) cell numbers in the blood. P values in panels c and d were calculated using unpaired, two-tailed t-tests.
Extended Data Fig. 8 CD19 and CD20 Expression Pre and Post LV20.19 CAR T-cell Therapy.
CD19 and CD20 expression on biopsy samples from patients progressing after LV20.19 CAR T-cell therapy compared to baseline sample obtained either at diagnosis or from a relapse sample prior to LV20.19 therapy. Dose level, time to relapse and day 28 response are described. Flow cytometric, mean fluorescence intensity, or immunohistochemistry values are presented as available from tissue material. All relapsing patients after LV20.19 CAR T-cells retained either CD19 or CD20 expression on tumor cells.
Extended Data Fig. 9 Detailed Analysis from Tumor Samples.
Small numbers of CAR T-cells were present in biopsy material of Patient #3 at day 34 post-infusion, and malignant B-cells appeared to inhibit CAR T-cell function. (a) Flow cytometric histograms of peripheral blood mononuclear cells (left column) and biopsy material (right column) analyzed for B cell content and surface CAR expression (Protein L). Peripheral blood CD4 and CD8 T-cells were gated on CD3 expression. The red arrows indicate surface Ig+ lymphoma cells. The same flow cytometry gating strategy shown in Supplementary Figure 8 was used to determine Protein L + cells (b) Cryopreserved LV20.19 CAR T-cells from the original infusion were thawed and tested for cytolytic activity versus 51Cr-labeled Raji lymphoma (CD19 + , CD20 + ) cells, U2OS cells (CD19-, CD20-), or biopsy cells at the effector-to-target (E:T) cell ratios indicated. Values represent specific lysis ± standard deviation. (c) Cryopreserved LV20.19 CAR T-cells from the original infusion were thawed, incubated with the indicated stimulator cells (Raji or biopsy cells) or staphylococcal enterotoxin B (SEB; positive control) for 6 h in the presence of brefeldin A, and analyzed by flow cytometry for IFNg. Percentages of IFNg+ CD4 and CD8 cells are indicated. The flow cytometry gating strategy for these assays involved setting a debris-free gate from FSC-A x SSC-A plots, followed by a CD4 or CD8 gate.
Extended Data Fig. 10 B-cell Persistence in Peripheral Blood of Patients Following LV20.19 CAR T-cell Infusion.
B-cell persistence in the blood of patients following infusion. Peripheral blood mononuclear cells collected from individual patients at the indicated times post-infusion were analyzed by flow cytometry for expression of CD19 (solid black lines) and CD20 (red lines). The percentages of CAR + T-cells in the CD3 compartment are also plotted for each patient (blue lines). These markers were analyzed from debris-free FSC-A x SSC-A gates. The Day 28 clinical response status for each patient is shown at the top. (a) Patients (n = 13) who received their goal dose of CAR T-cells had either low or nondetectable frequencies of B cells and then have remained B cell aplastic through follow-up to date. (b) Patients (n = 3) whose circulating B cells were not present at the time of infusion but reappeared as CAR T-cell numbers fell. (c) Patients (n = 3) that had large numbers of circulating lymphoma cells at the time of CAR T infusion. (d) Patients (n = 3) that had unique patterns of B cell aplasia and/or reappearance.
Supplementary information
Supplementary Information
Supplementary Note, Supplementary Figs. 1–8 and Supplementary Tables 1–5
Rights and permissions
About this article
Cite this article
Shah, N.N., Johnson, B.D., Schneider, D. et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med 26, 1569–1575 (2020). https://doi.org/10.1038/s41591-020-1081-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-020-1081-3
This article is cited by
-
Immune evasion in cell-based immunotherapy: unraveling challenges and novel strategies
Journal of Biomedical Science (2024)
-
Programmable synthetic receptors: the next-generation of cell and gene therapies
Signal Transduction and Targeted Therapy (2024)
-
Therapeutic options for large B-cell lymphoma relapsing after CD19-directed CAR T-cell therapy
Bone Marrow Transplantation (2024)
-
Proinflammatory polarization of engineered heat-inducible macrophages reprogram the tumor immune microenvironment during cancer immunotherapy
Nature Communications (2024)
-
Synthetic biology approaches for improving the specificity and efficacy of cancer immunotherapy
Cellular & Molecular Immunology (2024)