Abstract
Patients awaiting lung transplantation face high wait-list mortality, as injury precludes the use of most donor lungs. Although ex vivo lung perfusion (EVLP) is able to recover marginal quality donor lungs, extension of normothermic support beyond 6 h has been challenging. Here we demonstrate that acutely injured human lungs declined for transplantation, including a lung that failed to recover on EVLP, can be recovered by cross-circulation of whole blood between explanted human lungs and a Yorkshire swine. This xenogeneic platform provided explanted human lungs a supportive, physiologic milieu and systemic regulation that resulted in functional and histological recovery after 24 h of normothermic support. Our findings suggest that cross-circulation can serve as a complementary approach to clinical EVLP to recover injured donor lungs that could not otherwise be utilized for transplantation, as well as a translational research platform for immunomodulation and advanced organ bioengineering.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the text, figures and Supplementary Information.
References
World Health Organization. World Health Statistics 2017: Monitoring Health for the SDGs, Sustainable Development Goals (World Health Organization, 2017).
Chambers, D. C. et al. The international thoracic organ transplant registry of the International Society for Heart and Lung Transplantation: thirty-fifth adult lung and heart-lung transplant report—2018; focus theme: multiorgan transplantation. J. Heart Lung Transplant. 37, 1169–1183 (2018).
Van Herck, A., Verleden, S. E., Vanaudenaerde, B. M., Verleden, G. M. & Vos, R. Prevention of chronic rejection after lung transplantation. J. Thorac. Dis. 9, 5472–5488 (2017).
Sommer, W. et al. Extended criteria donor lungs and clinical outcome: results of an alternative allocation algorithm. J. Heart Lung Transplant. 32, 1065–1072 (2013).
Pinezich, M. & Vunjak-Novakovic, G. Bioengineering approaches to organ preservation ex vivo. Exp. Biol. Med. 244, 630–645 (2019).
Guenthart, B. A. et al. Cell replacement in human lung bioengineering. J. Heart Lung Transplant. 38, 215–224 (2019).
Yamada, K. et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of α-1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat. Med. 11, 32–34 (2005).
Sykes, M. & Sachs, D. H. Transplanting organs from pigs to humans. Sci. Immunol. 4, eaau6298 (2019).
Cypel, M. et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N. Engl. J. Med. 364, 1431–1440 (2011).
O’Neill, J. D. et al. Cross-circulation for extracorporeal support and recovery of the lung. Nat. Biomed. Eng. 1, 1–15 (2017).
Loor, G. et al. Prolonged EVLP using OCS lung: cellular and acellular perfusates. Transplantation 101, 2303–2311 (2017).
Sommer, W. et al. Prediction of transplant outcome after 24‐hour ex vivo lung perfusion using the organ care system in a porcine lung transplantation model. Am. J. Transplant. 19, 345–355 (2019).
Spratt, J. R. et al. An experimental study of the recovery of injured porcine lungs with prolonged normothermic cellular ex vivo lung perfusion following donation after circulatory death. Transpl. Int. J. 30, 932–944 (2017).
Guenthart, B. A. et al. Regeneration of severely damaged lungs using an interventional cross-circulation platform. Nat. Commun. 10, 1–16 (2019).
Hozain, A. E. et al. Multi-day maintenance of extracorporeal lungs using cross-circulation with conscious swine. J. Thorac. Cardiovasc. Surg. 159, 1640–1653 (2019).
Brigham, K. L. & Snell, J. D. In vivo assessment of pulmonary vascular integrity in experimental pulmonary edema. J. Clin. Invest. 52, 2041–2052 (1973).
de Perrot, M., Liu, M., Waddell, T. K. & Keshavjee, S. Ischemia-reperfusion-induced lung injury. Am. J. Respir. Crit. Care Med. 167, 490–511 (2003).
Slama, A. et al. Standard donor lung procurement with normothermic ex vivo lung perfusion: a prospective randomized clinical trial. J. Heart Lung Transplant. 36, 744–753 (2017).
Warnecke, G. et al. Normothermic ex-vivo preservation with the portable organ care system lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir. Med. 6, 357–367 (2018).
Loor, G. et al. Portable normothermic ex-vivo lung perfusion, ventilation, and functional assessment with the organ care system on donor lung use for transplantation from extended-criteria donors (EXPAND): a single-arm, pivotal trial. Lancet Respir. Med. 7, 975–984 (2019).
Andreasson, A. S. I. et al. Profiling inflammation and tissue injury markers in perfusate and bronchoalveolar lavage fluid during human ex vivo lung perfusion. Eur. J. Cardio-Thorac. Surg. 51, 577–586 (2017).
Okamoto, T., Wheeler, D., Farver, C. F. & McCurry, K. R. Transplant suitability of rejected human donor lungs with prolonged cold ischemia time in low-flow acellular and high-flow cellular ex vivo lung perfusion systems. Transplantation 103, 1799–1808 (2019).
Sadaria, M. R. et al. Cytokine expression profile in human lungs undergoing normothermic ex-vivo lung perfusion. Ann. Thorac. Surg. 92, 478–484 (2011).
Fujino, N. et al. Isolation of alveolar epithelial type II progenitor cells from adult human lungs. Lab. Investig. J. Tech. Methods Pathol. 91, 363–378 (2011).
Rock, J. R., Randell, S. H. & Hogan, B. L. M. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis. Model. Mech. 3, 545–556 (2010).
Tata, A. et al. Myoepithelial cells of submucosal glands can function as reserve stem cells to regenerate airways after injury. Cell Stem Cell 22, 668–683 (2018).
Olajuyin, A. M., Zhang, X. & Ji, H.-L. Alveolar type 2 progenitor cells for lung injury repair. Cell Death Discov. 5, 63 (2019).
Griesemer, A., Yamada, K. & Sykes, M. Xenotransplantation: immunological hurdles and progress toward tolerance. Immunol. Rev. 258, 241–258 (2014).
Breimer, M. E. et al. Extracorporeal (‘ex vivo’) connection of pig kidneys to humans. I. Clinical data and studies of platelet destruction. Xenotransplantation 3, 328–339 (1996).
Baquerizo, A. et al. Characterization of human xenoreactive antibodies in liver failure patients exposed to pig hepatocytes after bioartificial liver treatment: an ex vivo model of pig to human xenotransplantation 1,2. Transplantation 67, 5 (1999).
Levy, M. F. et al. Liver allotransplantation after extracorporeal hepatic support with transgenic (hCD55/hCD59) porcine livers: clinical results and lack of pig-to-human transmission of the porcine endogenous retrovirus 1. Transplantation 69, 272 (2000).
Li, Q. et al. Is sensitization to pig antigens detrimental to subsequent allotransplantation? Xenotransplantation 25, e12393 (2018).
Scheffert, J. L. & Raza, K. Immunosuppression in lung transplantation. J. Thorac. Dis. 6, 1039–1053 (2014).
Cochrane, C. G., Müller-Eberhard, H. J. & Aikin, B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J. Immunol. 105, 55–69 (1970).
OBERHOLZER, J. et al. Decomplementation with cobra venom factor prolongs survival of xenografted islets in a rat to mouse model. Immunology 97, 173–180 (1999).
Haihua, C., Wei, W., Kun, H., Yuanli, L. & Fei, L. Cobra venom factor-induced complement depletion protects against lung ischemia reperfusion injury through alleviating blood-air barrier damage. Sci. Rep. 8, 1–8 (2018).
Gorsuch, W. B., Guikema, B. J., Fritzinger, D. C., Vogel, C.-W. & Stahl, G. L. Humanized cobra venom factor decreases myocardial ischemia reperfusion injury. Mol. Immunol. 47, 506–510 (2009).
Campos, M. M. et al. The role of migrating leukocytes in IL-1β-induced up-regulation of kinin B1 receptors in rats. Br. J. Pharmacol. 135, 1107–1114 (2002).
Bickel, M. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 64, 456–460 (1993).
De Perrot, M. et al. Interleukin-8 release during early reperfusion predicts graft function in human lung transplantation. Am. J. Respir. Crit. Care Med. 165, 211–215 (2002).
Andreasson, A. S. I. et al. The role of interleukin-1β as a predictive biomarker and potential therapeutic target during clinical ex vivo lung perfusion. J. Heart Lung Transplant. 36, 985–995 (2017).
Scheller, J., Chalaris, A., Schmidt-Arras, D. & Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta Mol. Cell Res. 1813, 878–888 (2011).
Halloran, P. F. et al. IFN-γ alters the pathology of graft rejection: protection from early necrosis. J. Immunol. 166, 7072–7081 (2001).
Kawut, S. M. et al. Soluble P-selectin and the risk of primary graft dysfunction after lung transplantation. Chest 136, 237–244 (2009).
Loss, M. et al. Acute vascular rejection is associated with systemic complement activation in a pig-to-primate kidney xenograft model. Xenotransplantation 7, 186–196 (2000).
McCurry, K. R. et al. Humoral responses to pig-to-baboon cardiac transplantation: implications for the pathogenesis and treatment of acute vascular rejection and for accommodation. Hum. Immunol. 58, 91–105 (1997).
Zuber, J. & Sykes, M. Mechanisms of mixed chimerism-based transplant tolerance. Trends Immunol. 38, 829–843 (2017).
Kuwaki, K. et al. Heart transplantation in baboons using α-1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat. Med. 11, 29–31 (2005).
Lee, K. et al. Engraftment of human iPS cells and allogeneic porcine cells into pigs with inactivated RAG2 and accompanying severe combined immunodeficiency. Proc. Natl Acad. Sci. USA 111, 7260–7265 (2014).
Pan, H. et al. Lymphodepletive effects of rabbit anti-pig thymocyte globulin in neonatal swines. Transpl. Immunol. 39, 74–83 (2016).
Bottino, R. et al. Safe use of anti-Cd154 monoclonal antibody in pig islet xenotransplantation in monkeys. Xenotransplantation 24, e12283 (2017).
Nottle, M. B. et al. Targeted insertion of an anti-CD2 monoclonal antibody transgene into the GGTA1 locus in pigs using Fok I-dCas9. Sci. Rep. 7, 1–8 (2017).
Boneva, R. S. & Folks, T. M. Xenotransplantation and risks of zoonotic infections. Ann. Med. 36, 504–517 (2004).
Noordergraaf, J. et al. Pathogen elimination and prevention within a regulated, designated pathogen free, closed pig herd for long-term breeding and production of xenotransplantation materials. Xenotransplantation 25, e12428 (2018).
Denner, J. The porcine virome and xenotransplantation. Virol. J. 14, 171 (2017).
Godehardt, A. W., Costa, M. R. & Tönjes, R. R. Review on porcine endogenous retrovirus detection assays—impact on quality and safety of xenotransplants. Xenotransplantation 22, 95–101 (2015).
Niu, D. et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science 357, 1303–1307 (2017).
Dieckhoff, B. et al. Knockdown of porcine endogenous retrovirus (PERV) expression by PERV-specific shRNA in transgenic pigs. Xenotransplantation 15, 36–45 (2008).
Andreasson, A. et al. The effect of ex vivo lung perfusion on microbial load in human donor lungs. J. Heart Lung Transplant. 33, 910–916 (2014).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinforma. Oxf. Engl. 30, 2114–2120 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinforma. Oxf. Engl. 29, 15–21 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Blighe, K., Rana, S. & Lewis, M. EnhancedVolcano: Publication-ready Volcano Plots with Enhanced Colouring and Labeling. Bioconductor version: release (3.10) https://doi.org/10.18129/B9.bioc.EnhancedVolcano (2019).
Subhash, S. & Kanduri, C. GeneSCF: a real-time based functional enrichment tool with support for multiple organisms. BMC Bioinf. 17, 365 (2016).
GTEx Consortium. The genotype-tissue expression (GTEx) project. Nat. Genet. 45, 580–585 (2013).
Acknowledgements
The authors thank the following collaborators and supporters: Institute of Comparative Medicine veterinary staff, including A. Hubbard, S. Robertson, R. Ober, A. McLuckie, N. Herndon, D. Ordanes and A. Rivas for supporting animal studies; Weill Cornell Microscopy and Image Analysis Core Facility staff, including L. Cohen-Gould and J. P. Jimenez for transmission electron microscopy imaging services; Rockefeller University Electron Microscopy Resource Center staff, including K. Uyru and N. Soplop for scanning electron microscopy imaging services; Herbert Irving Comprehensive Cancer Center Molecular Pathology Shared Resources, including T. Wu, D. Sun and R. Chen for histology services; S. Chicotka, P. Liou, M. Foley, J. Diaz, M.S. Fultz, J. Adcock, N. Llore, E. Lopes, G. Pierre and I. Fedoriv for technical and analytical support; S. Pistilli, K. Fragoso and S. Halligan for administrative support. The authors gratefully acknowledge funding support from the National Institutes of Health (EB27062, HL007854, HL120046, HL134760), Mikati Foundation and Blavatnik Foundation (STAR grant).
Author information
Authors and Affiliations
Contributions
A.E.H., J.D.O., R.D., A.D.G., B.A.G., G.V.-N. and M.B. designed the study. A.E.H., J.D.O., M.R.P., Y.T., R.D., K.M.C., A.T., K.F., R.U., M.S., D.Q., J.W.S., N.L.C., J.T., J.K., Y.-W.C., A.R., B.A.G. and M.B. performed experiments. C.C.M. performed the blinded pathologic assessment. A.E.H., J.D.O., M.R.P., Y.T., K.M.C., A.T., M.S., J.A.R., E.C.R., D.Q. and H.-W.S. analyzed data. A.E.H., J.D.O., G.V.-N. and M.B. co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
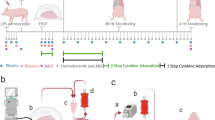
Extended Data Fig. 1 Experimental setup and xenogeneic cross-circulation circuit parameters.
a, Schematic of xenogeneic cross-circulation with mean values of perfusion circuit parameters. To maintain pulmonary vein drainage, lungs were positioned approximately 10 cm higher than swine hosts (Δh). b, Immunosuppression regimen, including induction immunosuppression before cross-circulation and maintenance immunosuppression during cross-circulation. c, Experimental setup during xenogeneic cross-circulation procedure. d, Perfusion circuit connecting the vascular compartments of explanted human lungs and anesthetized swine host. e, Pulmonary artery and vein cannulas connecting explanted human lungs to the xenogeneic cross-circulation circuit. f, Swine host neck cannulation sites. Extracorporeal circuit parameters: g, Pressure. h, Flow. i, Temperature. All graphs represent data for human lungs (n = 5 independent experiments). All values represent mean ± standard deviation.
Extended Data Fig. 2 Multi-scale analyses of human lung 1.
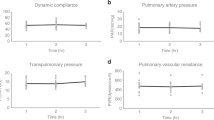
a, Gross photography. b, Radiography. c, Bronchoscopy of left and right lung. d, PaO2/FiO2. e, Change in pO2 (Δp= |pPA – pPV|). f, Change in pCO2 (Δp= |pPA – pPV|). g, Lung weight. h, Dynamic compliance. i, Peak inspiratory pressure (PIP). j, Transpulmonary pressure gradient (TPG). k, Lactate. All graphs represent images and data for human lung 1 (n = 1 independent experiment). e-i, k, Data points represent a single value obtained at each time point. j, Data points represent mean ± standard deviation of all values obtained at each time point.
Extended Data Fig. 3 Multi-scale analyses of human lung 2.
a, Gross photography. b, Radiography. c, Bronchoscopy of left and right lung. d, PaO2/FiO2. e, Change in pO2 (Δp= |pPA – pPV|). f, Change in pCO2 (Δp= |pPA – pPV|). g, Lung weight. h, Dynamic compliance. i, Peak inspiratory pressure (PIP). j, Transpulmonary pressure gradient (TPG). k, Lactate. All graphs represent images and data for human lung 2 (n = 1 independent experiment). e-i, k, All data points represent a single value obtained at each time point. j, Data points represent mean ± standard deviation of all values obtained at each time point.
Extended Data Fig. 4 Multi-scale analyses of human lung 3.
a, Gross photography. b, Radiography. c, Bronchoscopy of left and right lung. d, PaO2/FiO2. e, Change in pO2 (Δp= |pPA – pPV|). f, Change in pCO2 (Δp= |pPA – pPV|). g, Lung weight. h, Dynamic compliance. i, Peak inspiratory pressure (PIP). j, Transpulmonary pressure gradient (TPG). k, Lactate. All graphs represent images and data for human lung 3 (n = 1 independent experiment). e-i, k, All data points represent a single value obtained at each time point. j, Data points represent mean ± standard deviation of all values obtained at each time point.
Extended Data Fig. 5 Multi-scale analyses of human lung 4.
a, Gross photography. b, Bronchoscopy. c, Histologic staining with hematoxylin and eosin. d, PaO2/FiO2. e, Change in pO2 (Δp= |pPA – pPV|). f, Change in pCO2 (Δp= |pPA – pPV|). g, Lung weight. h, Dynamic compliance. i, Peak inspiratory pressure (PIP). j, Transpulmonary pressure gradient (TPG). k, Lactate. All graphs represent images and data for human lung 4 (n = 1 independent experiment). d-k, All data points represent a single value obtained at each time point.
Extended Data Fig. 6 Multi-scale analyses of human lung 5.
a, Gross photography. b, Bronchoscopy. c, Histologic staining with hematoxylin and eosin. d, PaO2/FiO2. e, Change in pO2 (Δp= |pPA – pPV|). f, Change in pCO2 (Δp= |pPA – pPV|). g, Lung weight. h, Dynamic compliance. i, Peak inspiratory pressure (PIP). j, Transpulmonary pressure gradient (TPG). k, Lactate. All graphs represent images and data for human lung 5 (n = 1 independent experiment). d-k, All data points represent a single value obtained at each time point.
Extended Data Fig. 7 Histologic evaluation of human lung 1.
Micrographs of hematoxylin and eosin staining of upper and lower lobes. a, Lung parenchyma at low magnification. b, Lung parenchyma at high magnification. c, Small airways. d, Pulmonary vessels. e, Scanning electron micrographs of alveoli. f, Transmission electron micrographs of alveolar septa. Immunohistochemical staining of: g, HT2-280+ type II pneumocytes. h, Caveolin-1+ type I pneumocytes. i, CC10+ club cells and Mucin 5B+ goblet cells. j, α-tubulin+ ciliated cells. k, α-SMA+ submucosal glands. l, p63+ basal cells. m, CD31+ microvascular endothelial cells and ZO-3+ epithelial tight junctions. n, Vascular endothelial (VE)-Cadherin+ endothelial cells.
Extended Data Fig. 8 Histologic evaluation of human lung 2.
Micrographs of hematoxylin and eosin staining of upper and lower lobes: a, Lung parenchyma at low magnification. b, Lung parenchyma at high magnification. c, Small airways. d, Pulmonary vessels. e, Scanning electron micrographs of alveoli. f, Transmission electron micrographs of alveolar septa. Immunohistochemical staining of: g, HT2-280+ type II pneumocytes. h, Caveolin-1+ type I pneumocytes. i, CC10+ club cells and Mucin 5B+ goblet cells. j, α-tubulin+ ciliated cells. k, CD31+ microvascular endothelial cells and ZO-3+ epithelial tight junctions. l, Vascular endothelial (VE)-Cadherin+ endothelial cells.
Extended Data Fig. 9 Histologic evaluation of human lung 3.
Micrographs of hematoxylin and eosin staining of upper and lower lobes: a, Lung parenchyma at low magnification. b, Lung parenchyma at high magnification. c, Small airways. d, Pulmonary vessels. e, Scanning electron micrographs of alveoli. f, Transmission electron micrographs of alveolar septa. Immunohistochemical staining of: g, HT2-280+ type II pneumocytes. h, Caveolin-1+ type I pneumocytes. i, CC10+ club cells and Mucin 5B+ goblet cells. j, α-tubulin+ ciliated cells. k, CD31+ microvascular endothelial cells and ZO-3+ epithelial tight junctions. l, Vascular endothelial (VE)-Cadherin+ endothelial cells.
Extended Data Fig. 10 Envisioned applications of xenogeneic cross-circulation platform.
a, Clinical applications of human lungs recovered using xenogeneic cross-circulation. Injured human lungs can be recovered at organ recovery or transplant centers. Lungs recovered by cross-circulation could be transplanted into recipient patients awaiting transplantation. b, Research applications. Xenogeneic cross-circulation can be used as a physiologic bioreactor to maintain extracorporeal organs or grafts, enabling research and development of bioengineered constructs, advanced therapeutics, disease models, and investigation of cross-species immunological interactions. EVLP, ex vivo lung perfusion. XC, cross-circulation.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5 and Supplementary Tables 1–14.
Supplementary Video 1
Evaluation of human lung recovery throughout 24 h xenogeneic cross-circulation.
Supplementary Video 2
Ventilation and recruitment of human lungs during 24 h of xenogeneic cross-circulation.
Supplementary Video 3
Live uptake of surfactant protein B by human lungs after 24 h of xenogeneic cross-circulation.
Supplementary Data 1
RNA sequencing raw data.
Rights and permissions
About this article
Cite this article
Hozain, A.E., O’Neill, J.D., Pinezich, M.R. et al. Xenogeneic cross-circulation for extracorporeal recovery of injured human lungs. Nat Med 26, 1102–1113 (2020). https://doi.org/10.1038/s41591-020-0971-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-020-0971-8
This article is cited by
-
Novel Strategies for Optimization of the Pre-transplant Donor Lung
Current Pulmonology Reports (2024)
-
Crystal ribcage: a platform for probing real-time lung function at cellular resolution
Nature Methods (2023)
-
Reduction of primary graft dysfunction using cytokine adsorption during organ preservation and after lung transplantation
Nature Communications (2022)
-
3D Printing: Advancement in Biogenerative Engineering to Combat Shortage of Organs and Bioapplicable Materials
Regenerative Engineering and Translational Medicine (2022)
-
Ex vivo repair of human donor lungs for transplantation
Nature Medicine (2020)