Abstract
Neutralizing antibodies to adeno-associated virus (AAV) vectors are highly prevalent in humans1,2, and block liver transduction3,4,5 and vector readministration6; thus, they represent a major limitation to in vivo gene therapy. Strategies aimed at overcoming anti-AAV antibodies are being studied7, which often involve immunosuppression and are not efficient in removing pre-existing antibodies. Imlifidase (IdeS) is an endopeptidase able to degrade circulating IgG that is currently being tested in transplant patients8. Here, we studied if IdeS could eliminate anti-AAV antibodies in the context of gene therapy. We showed efficient cleavage of pooled human IgG (intravenous Ig) in vitro upon endopeptidase treatment. In mice passively immunized with intravenous Ig, IdeS administration decreased anti-AAV antibodies and enabled efficient liver gene transfer. The approach was scaled up to nonhuman primates, a natural host for wild-type AAV. IdeS treatment before AAV vector infusion was safe and resulted in enhanced liver transduction, even in the setting of vector readministration. Finally, IdeS reduced anti-AAV antibody levels from human plasma samples in vitro, including plasma from prospective gene therapy trial participants. These results provide a potential solution to overcome pre-existing antibodies to AAV-based gene therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All requests for raw and analyzed data and materials will be promptly reviewed by Généthon, Institut National de la Santé et de la Recherche Médicale or Spark Therapeutics to verify if the request is subject to any intellectual property or confidentiality obligations. Patient-related data not included in the manuscript were generated as part of a clinical trial and are not available because of patient confidentiality issues. Any data and materials that can be shared will be released via a material transfer agreement established with the relevant institution that generated the data or owns the materials. Source data for Figs. 1–4 and Extended Data Figs. 1–7 are included with this paper.
References
Boutin, S. et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 21, 704–712 (2010).
Li, C. et al. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther. 19, 288–294 (2012).
Jiang, H. et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood 108, 3321–3328 (2006).
Manno, C. S. et al. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat. Med. 12, 342–347 (2006).
Scallan, C. D. et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood 107, 1810–1817 (2006).
Mingozzi, F. & High, K. A. Overcoming the host immune response to adeno-associated virus gene delivery vectors: the race between clearance, tolerance, neutralization, and escape. Annu. Rev. Virol. 4, 511–534 (2017).
Meliani, A. et al. Antigen-selective modulation of AAV immunogenicity with tolerogenic rapamycin nanoparticles enables successful vector re-administration. Nat. Commun. 9, 4098 (2018).
Jordan, S. C. et al. IgG endopeptidase in highly sensitized patients undergoing transplantation. N. Engl. J. Med. 377, 442–453 (2017).
George, L. A. et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N. Engl. J. Med. 377, 2215–2227 (2017).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377, 1713–1722 (2017).
Miesbach, W. et al. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood 131, 1022–1031 (2018).
Rangarajan, S. et al. AAV5-factor VIII gene transfer in severe hemophilia A. N. Engl. J. Med. 377, 2519–2530 (2017).
von Pawel-Rammingen, U., Johansson, B. P. & Björck, L. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 21, 1607–1615 (2002).
Kizlik-Masson, C. et al. Cleavage of anti-PF4/heparin IgG by a bacterial protease and potential benefit in heparin-induced thrombocytopenia. Blood 133, 2427–2435 (2019).
Meliani, A. et al. Determination of anti-adeno-associated virus vector neutralizing antibody titer with an in vitro reporter system. Hum. Gene Ther. Methods 26, 45–53 (2015).
Agniswamy, J., Lei, B., Musser, J. M. & Sun, P. D. Insight of host immune evasion mediated by two variants of group A Streptococcus Mac protein. J. Biol. Chem. 279, 52789–52796 (2004).
Mingozzi, F. et al. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci. Transl. Med. 5, 194ra92 (2013).
Winstedt, L. et al. Complete removal of extracellular IgG antibodies in a randomized dose-escalation phase I study with the bacterial enzyme IdeS: a novel therapeutic opportunity. PLoS ONE 10, e0132011 (2015).
Lorant, T. et al. Safety, immunogenicity, pharmacokinetics, and efficacy of degradation of anti-HLA antibodies by IdeS (imlifidase) in chronic kidney disease patients. Am. J. Transplant. 18, 2752–2762 (2018).
Gao, G.-P. et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl Acad. Sci. USA 99, 11854–11859 (2002).
Mingozzi, F. et al. Pharmacological modulation of humoral immunity in a nonhuman primate model of AAV gene transfer for hemophilia B. Mol. Ther. 20, 1410–1416 (2012).
Nathwani, A. C. et al. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol. Ther. 19, 876–885 (2011).
Lisowski, L. et al. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature 506, 382–386 (2014).
McIntosh, J. et al. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood 121, 3335–3344 (2013).
Aronson, S. J. et al. Prevalence and relevance of pre-existing anti-adeno-associated virus immunity in the context of gene therapy for Crigler–Najjar syndrome. Hum. Gene Ther. 30, 1297–1305 (2019).
Puzzo, F. et al. Rescue of Pompe disease in mice by AAV-mediated liver delivery of secretable acid α-glucosidase. Sci. Transl. Med. 9, eaam6375 (2017).
Mingozzi, F. et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood 110, 2334–2341 (2007).
Acknowledgements
We thank the staff of the Nantes-Atlantic National College of Veterinary Medicine, Food Science and Engineering and Virscio staff for their help in designing and performing the NHP studies. We also thank the Spark Therapeutics vector production, bioanalytical, immunology, liver, sample management and laboratory information management teams for their contribution to the NHP studies. The work was supported by the Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, Sorbonne Université.
Author information
Authors and Affiliations
Contributions
C.L., E.B., J.M.A., S.D., H.H., F.C., S.M., D.L., J.S., K.H., L.V.W., B.M., A.M., A.F., V.D., D.M.C. and H.B. performed the experiments. C.L., G.R., S.M.A., S.L.-D. and F.M. analyzed the results and wrote the manuscript. X.M.A., S.M.A., S.L.-D. and F.M. conceived and supervised the studies.
Corresponding authors
Ethics declarations
Competing interests
J.M.A., H.H., S.M., D.L., J.S., K.H., D.M.C., H.B., X.M.A., S.M.A. and F.M. are employees of Spark Therapeutics, a Roche company. F.M., S.L.D., S.M.A. and C.L. are inventors on a patent related to this work. Patent applicants: Institut National de la Santé et de la Recherche Médicale; Généthon; Sorbonne Université; Université Paris Descartes; Université Paris Diderot; Spark Therapeutics. Inventors: S. Lacroix-Desmazes, F. Mingozzi, C. Leborgne, J. D. Dimitrov, S. M. Armour. Application no. PCT/EP2019/069280 (application request filed). Specific aspects of the manuscript covered in the patent application include use of IdeS to decrease pre-existing antibody immunity to AAV and allow for vector readministration.
Additional information
Peer review informaton Kate Gao was the primary editor on this article, and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
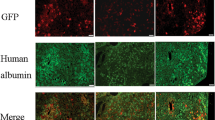
Extended Data Fig. 1 IdeS degrades anti-AAV antibodies and allows successful liver transduction in mice passively immunized with IVIg.
a, IVIg was incubated in vitro with saline (PBS), commercially sourced IdeS (IdeS-C), or lab-made IdeS (IdeS) for 24 hours prior to measurement of anti-AAV8 NAb titers (n = 1 per condition tested in triplicate, one representative out of two independent experiments shown). b, effect of IdeS on AAV8 vector transduction efficiency of HEK293 cells. An AAV8-Luc vector was incubated for 24 hours at 37 °C with PBS (- IdeS) or with increasing amounts of IdeS (+ IdeS). Control, AAV8-Luc not incubated at 37 °C. Relative Light Units (RLU) measured in duplicate at increasing multiplicity of infection (MOI). n = 1 per condition tested in duplicate, one representative out of two independent experiments shown. c, Plasma concentration of IdeS over time in non-human primates (n = 2 animals, duplicate independent measurements of each sample). The inset shows the estimation of the half-life of the enzyme. d, vector genome copy number (VGNC) in passively immunized C57BL/6 mice injected with an AAV8-GLuc vector (n = 6 mice per group, one representative out of two independent experiments shown). e, f, Effect of IdeS on anti-AAV8 IgG e, and NAb titers f, measured 24 hours after treatment in passively immunized HB mice (n = 4 mice per group, data derived from one experiment). g, vector genome copy number (VGNC) in HB mice injected with an AAV8-hFIX vector. All data are shown as mean ± s.d. Statistical analyses were performed by d, one-way ANOVA with Tukey’s multiple comparisons test; e, g, one-way ANOVA with Tukey’s multiple comparisons test; f, one-way ANOVA, non-parametric with Dunn’s multiple comparisons test.
Extended Data Fig. 2 IdeS treatment enhances transduction in NHPs in the presence of pre-existing anti-AAV Nabs.
a, Follow-up of digestion of circulating IgG in NHP6 (IdeS treated) and NHP2 (control) assessed by Western blot. One representative out of two independent experiments shown. b, Effect of IdeS administration on anti-AAV8 IgG and c, NAbs (n = 1 monkey per treatment group, triplicate independent measurements of each sample). The horizontal dotted lines in panels b, and c, represent the levels of anti-AAV8 IgG and NAbs in the control animal NHP2. d, hFIX transgene levels in plasma over time (n = 1 monkey per treatment group, duplicate independent measurements of each sample). e, vector genome copy number (VGCN) in liver at sacrifice (n = 1 monkey per treatment group, average VGCN in each of the four main liver sections). f, Anti-AAV8 IgG and g, anti-AAV8 NAbs at baseline and post AAV8-hFIX vector administration (n = 1 monkey per treatment group, duplicate independent measurements of each sample). All data are shown as mean ± s.d. Statistical analyses were performed by b, c, repeated measures one-way ANOVA with Dunnett’s multiple comparisons test (vs. Day-2); d, f, g, repeated measures two-way ANOVA with Sidak’s multiple comparisons test (vs. NHP2); e, two-tailed unpaired t-test.
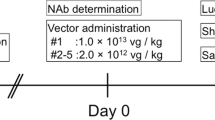
Extended Data Fig. 3 IdeS treatment allows for AAV8 vector readministration in NHPs.
Two male Cynomolgus monkeys with an anti-AAV8 NAb titer of 1:31.6 received an AAV8-hFIX vector (5 × 1012 vg kg−1) at day 0, with (NHP4, treated) or without (NHP1, control) an intravenous infusion of IdeS (500 µg kg−1) at day -1. At day 82 and 83, both NHPs received IdeS twice (500 µg kg−1 per infusion) followed by a second administration of the AAV8-hFIX vector (5 × 1012 vg kg−1) at day 84. b, Anti-AAV8 IgG measured over time in NHP4 after the first IdeS administration (n = 1 monkey per treatment group, triplicate independent measurements of each sample). The horizontal dotted line represents the levels of anti-AAV8 IgG in the control animal NHP1 (see Supplementary Table 1). c, hFIX in plasma and c, anti-AAV8 IgG at baseline and after the first vector administration (n = 1 monkey per treatment group, duplicate independent measurements of each sample). d, hFIX in plasma following AAV8-hFIX readministration (n = 1 monkey per treatment group, duplicate independent measurements of each sample). e, Western blot follow-up of total IgG cleavage between day 82 and 105 after the second IdeS treatment in NHP1 and NHP4. One representative out of two independent experiments shown. f, Anti-AAV8 IgG and g, NAbs measured over time in NHP1 and NHP4 after the second IdeS administration (n = 1 monkey per treatment group, duplicate independent measurements of each sample). All data are shown as mean ± s.d. Statistical analyses were performed by b, repeated measures one-way ANOVA with Dunnett’s multiple comparisons test (vs. Day -1); c,d, repeated measures two-way ANOVA with Sidak’s multiple comparisons test (vs. NHP1); f, repeated measures one-way ANOVA with Dunnett’s multiple comparisons test (vs. day -82), g, repeated measures, non-parametric one-way ANOVA with Dunn’s multiple comparisons test (vs. day -82).
Extended Data Fig. 4 IdeS treatment allows for AAV8 vector readministration in NHPs.
a, hFIX in plasma following AAV8-hFIX readministration. b, Anti-hFIX IgG measured by ELISA. c, vector genome copy number (VGCN) in liver at sacrifice (n = 1 monkey per treatment group, average VGCN in each of the four main liver sections). d, Anti-AAV8 IgG and e, anti-AAV8 NAbs pre- and post- AAV8-hFIX vector readministration. f, Anti-AAV8 IgM measured over time in NHP1 and NHP4 after the second IdeS administration. a, b, d–f, n = 1 monkey per treatment group, duplicate independent measurements of each sample. All data are shown as mean ± s.d. Statistical analyses were performed by a, b, d–f repeated measures two-way ANOVA with Sidak’s multiple comparisons test (vs. NHP1); c, two-tailed unpaired t-test.
Extended Data Fig. 5 Follow up of antibody responses following vector readministration.
a, Change in anti-AAV-LK03 NAb titers following IdeS administration. b,c, kinetics of plasma anti-AAV-LK03 IgM titers in animals from the PBS (b) and IdeS (c) treatment groups. d,e, kinetics of anti-AAV-LK03 IgG titers in animals from the PBS (d) and IdeS (e) treatment groups. f,g, Kinetics of anti-hFVIII IgG titers in animals from the PBS (f) and IdeS (g) treatment groups. Individual animals are shown in each graph.
Extended Data Fig. 6 Effect of IdeS on total IgG and anti-IdeS IgG in NHPs.
A total of 6 NHPs were included in this evaluation. NHP2 and NHP3 received a course of IdeS at day 37 post vector delivery a, total IgG measured before and, two and fifteen days after IdeS injections (n = 6 animals, in the box plot, the box extends from the 25th to 75th percentiles, whiskers show minima and maxima and median is shown in the box). b, development of anti-IdeS antibodies in NHP, 15 days after IdeS injections. c, prevalence of anti-IdeS antibodies in a cohort of 52 human serum samples. Samples were divided in [anti-IdeS]<5 μg/ml (seronegative), [anti-IdeS]<50 μg/ml (seropositive), [anti-IdeS]>50μg/ml (high seropositive). Statistical analyses were performed by a, one-way ANOVA with Dunnett’s multiple comparisons test (vs. Day 0); b, two-tailed, paired Wilcoxon test.
Extended Data Fig. 7 Effect of anti-IdeS antibodies on IgG digestion.
Serum samples from 2 Cynomolgus monkeys NHP1 (a, b) and NHP6 (c, d) collected at day -7 prior to IdeS treatment and at 21 days after were incubated with IdeS for up to 24 hours (one representative out of two independent experiments shown). a.,Western blot showing degradation of IgG over time and b, quantification of the Fc fragment of IgG in NHP1. c, Western blot showing degradation of IgG over time and d, quantification of the Fc fragment of IgG in NHP6.
Supplementary information
Supplementary Information
Supplementary Tables 1–5
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 1
Unprocessed Western Blots
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 2
Unprocessed Western Blots
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 4
Unprocessed Western Blots
Source Data Extended Data Fig. 1
Statistical Source Data
Source Data Extended Data Fig. 2
Statistical Source Data
Source Data Extended Data Fig. 2
Unprocessed Western Blots
Source Data Extended Data Fig. 3
Statistical Source Data
Source Data Extended Data Fig. 3
Unprocessed Western Blots
Source Data Extended Data Fig. 4
Statistical Source Data
Source Data Extended Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig. 6
Statistical Source Data
Source Data Extended Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 7
Unprocessed Western Blots
Rights and permissions
About this article
Cite this article
Leborgne, C., Barbon, E., Alexander, J.M. et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat Med 26, 1096–1101 (2020). https://doi.org/10.1038/s41591-020-0911-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-020-0911-7
This article is cited by
-
tRNA therapeutics for genetic diseases
Nature Reviews Drug Discovery (2024)
-
Adeno-associated virus as a delivery vector for gene therapy of human diseases
Signal Transduction and Targeted Therapy (2024)
-
Prednisolone and rapamycin reduce the plasma cell gene signature and may improve AAV gene therapy in cynomolgus macaques
Gene Therapy (2024)
-
Multicenter assessment and longitudinal study of the prevalence of antibodies and related adaptive immune responses to AAV in adult males with hemophilia
Gene Therapy (2024)
-
Harnessing whole human liver ex situ normothermic perfusion for preclinical AAV vector evaluation
Nature Communications (2024)