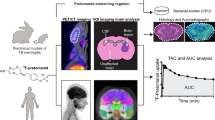
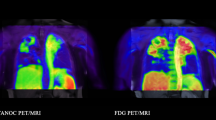
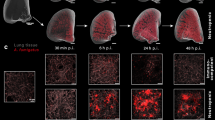
Abstract
Tuberculosis (TB) is the leading cause of death from a single infectious agent, requiring at least 6 months of multidrug treatment to achieve cure1. However, the lack of reliable data on antimicrobial pharmacokinetics (PK) at infection sites hinders efforts to optimize antimicrobial dosing and shorten TB treatments2. In this study, we applied a new tool to perform unbiased, noninvasive and multicompartment measurements of antimicrobial concentration–time profiles in humans3. Newly identified patients with rifampin-susceptible pulmonary TB were enrolled in a first-in-human study4 using dynamic [11C]rifampin (administered as a microdose) positron emission tomography (PET) and computed tomography (CT). [11C]rifampin PET–CT was safe and demonstrated spatially compartmentalized rifampin exposures in pathologically distinct TB lesions within the same patients, with low cavity wall rifampin exposures. Repeat PET–CT measurements demonstrated independent temporal evolution of rifampin exposure trajectories in different lesions within the same patients. Similar findings were recapitulated by PET–CT in experimentally infected rabbits with cavitary TB and confirmed using postmortem mass spectrometry. Integrated modeling of the PET-captured concentration–time profiles in hollow-fiber bacterial kill curve experiments provided estimates on the rifampin dosing required to achieve cure in 4 months. These data, capturing the spatial and temporal heterogeneity of intralesional drug PK, have major implications for antimicrobial drug development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analyzed in this study are included in this article and its Supplementary Information.
References
World Health Organization. Global Tuberculosis Report 2019 (World Health Organization, 2019).
Reynolds, J. & Heysell, S. K. Understanding pharmacokinetics to improve tuberculosis treatment outcome. Expert Opin. Drug Metab. Toxicol. 10, 813–823 (2014).
DeMarco, V. P. et al. Determination of [11C]rifampin pharmacokinetics within Mycobacterium tuberculosis-infected mice by using dynamic positron emission tomography bioimaging. Antimicrob. Agents Chemother. 59, 5768–5774 (2015).
Tucker, E. W. et al. Noninvasive 11C-rifampin positron emission tomography reveals drug biodistribution in tuberculous meningitis. Sci. Transl. Med. 10, eaau0965 (2018).
Diacon, A. H. et al. Early bactericidal activity of high-dose rifampin in patients with pulmonary tuberculosis evidenced by positive sputum smears. Antimicrob. Agents Chemother. 51, 2994–2996 (2007).
Chigutsa, E. et al. Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob. Agents Chemother. 59, 38–45 (2015).
Pasipanodya, J. G. et al. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J. Infect. Dis. 208, 1464–1473 (2013).
Swaminathan, S. et al. Drug concentration thresholds predictive of therapy failure and death in children with tuberculosis: bread crumb trails in random forests. Clin. Infect. Dis. 63, S63–S74 (2016).
Grobbelaar, M. et al. Evolution of rifampicin treatment for tuberculosis. Infect. Genet. Evol. 74, 103937 (2019).
Boeree, M. J. et al. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect. Dis. 17, 39–49 (2017).
Svensson, R. J. et al. Greater early bactericidal activity at higher rifampicin doses revealed by modeling and clinical trial simulations. J. Infect. Dis. 218, 991–999 (2018).
Pasipanodya, J. G. et al. Artificial intelligence-derived 3-way concentration-dependent antagonism of gatifloxacin, pyrazinamide, and rifampicin during treatment of pulmonary tuberculosis. Clin. Infect. Dis. 67, S284–S292 (2018).
Ehrlich, P. Address in pathology on chemotherapeutics: scientific principles, methods, and results. Lancet 182, 445–451 (1913).
Velasquez, G. E. et al. Efficacy and safety of high-dose rifampin in pulmonary tuberculosis: a randomized controlled trial. Am. J. Respir. Crit. Care Med. 198, 657–666 (2018).
Aarnoutse, R. E. et al. Pharmacokinetics, tolerability, and bacteriological response of rifampin administered at 600, 900, and 1,200 milligrams daily in patients with pulmonary tuberculosis. Antimicrob. Agents Chemother. 61, e01054-17 (2017).
Peloquin, C. A. et al. Pharmacokinetic evidence from the HIRIF trial to support increased doses of rifampin for tuberculosis. Antimicrob. Agents Chemother. 61, e01054-17 (2017).
Te Brake, L. H. M., Boeree, M. J. & Aarnoutse, R. E. Conflicting findings on an intermediate dose of rifampicin for pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 199, 1166–1167 (2019).
Dorman, S. E. et al. Substitution of rifapentine for rifampin during intensive phase treatment of pulmonary tuberculosis: study 29 of the Tuberculosis Trials Consortium. J. Infect. Dis. 206, 1030–1040 (2012).
Prideaux, B. et al. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat. Med. 21, 1223–1227 (2015).
Dheda, K. et al. Drug-penetration gradients associated with acquired drug resistance in patients with tuberculosis. Am. J. Respir. Crit. Care Med. 198, 1208–1219 (2018).
Hunter, R. L. The pathogenesis of tuberculosis: the early infiltrate of post-primary (adult pulmonary) tuberculosis: a distinct disease entity. Front. Immunol. 9, 2108 (2018).
Jain, S. K. et al. Tuberculous meningitis: a roadmap for advancing basic and translational research. Nat. Immunol. 19, 521–525 (2018).
Pan, H. et al. Ipr1 gene mediates innate immunity to tuberculosis. Nature 434, 767–772 (2005).
Nau, R. et al. Penetration of rifampicin into the cerebrospinal fluid of adults with uninflamed meninges. J. Antimicrob. Chemother. 29, 719–724 (1992).
Urbanowski, M. E. et al. Repetitive aerosol exposure promotes cavitary tuberculosis and enables screening for targeted inhibitors of extensive lung destruction. J. Infect. Dis. 218, 53–63 (2018).
Canetti, G. Present aspects of bacterial resistance in tuberculosis. Am. Rev. Respir. Dis. 92, 687–703 (1965).
Benator, D. et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet 360, 528–534 (2002).
Kjellsson, M. C. et al. Pharmacokinetic evaluation of the penetration of antituberculosis agents in rabbit pulmonary lesions. Antimicrob. Agents Chemother. 56, 446–457 (2012).
Rifat, D. et al. Pharmacokinetics of rifapentine and rifampin in a rabbit model of tuberculosis and correlation with clinical trial data. Sci. Transl. Med. 10, eaai7786 (2018).
Le Guellec, C., Gaudet, M. L., Lamanetre, S. & Breteau, M. Stability of rifampin in plasma: consequences for therapeutic monitoring and pharmacokinetic studies. Ther. Drug Monit. 19, 669–674 (1997).
Samara, E. et al. Antibiotic stability over six weeks in aqueous solution at body temperature with and without heat treatment that mimics the curing of bone cement. Bone Joint Res. 6, 296–306 (2017).
Magombedze, G. et al. Transformation morphisms and time-to-extinction analysis that map therapy duration from preclinical models to patients with tuberculosis: translating from apples to oranges. Clin. Infect. Dis. 67, S349–S358 (2018).
Lappin, G., Noveck, R. & Burt, T. Microdosing and drug development: past, present and future. Expert Opin. Drug Metab. Toxicol. 9, 817–834 (2013).
Nix, D. E., Goodwin, S. D., Peloquin, C. A., Rotella, D. L. & Schentag, J. J. Antibiotic tissue penetration and its relevance: impact of tissue penetration on infection response. Antimicrob. Agents Chemother. 35, 1953–1959 (1991).
Liu, L. et al. Radiosynthesis and bioimaging of the tuberculosis chemotherapeutics isoniazid, rifampicin and pyrazinamide in baboons. J. Med. Chem. 53, 2882–2891 (2010).
Ordonez, A. A. et al. Molecular imaging of bacterial infections: overcoming the barriers to clinical translation. Sci. Transl. Med. 11, eaax8251 (2019).
Rubinstein, L. V. et al. The statistics of phase 0 trials. Stat. Med. 29, 1072–1076 (2010).
Ordonez, A. A. et al. Radiosynthesis and PET bioimaging of 76Br-bedaquiline in a murine model of tuberculosis. ACS Infect. Dis. 5, 1996–2020 (2019).
US Food and Drug Administration. Guidance for Industry, Investigators, and Reviewers: Exploratory IND Studies (US Food and Drug Administration, 2006).
US Food and Drug Administration. Guidance for Industry and Researchers: The Radioactive Drug Research Committee: Human Research Without an Investigational New Drug Application (US Food and Drug Administration, 2010).
US Food and Drug Administration. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers (Center for Drug Evaluation and Research, 2005).
Hedlund, L. W., Vock, P. & Effmann, E. L. Evaluating lung density by computed tomography. Semin. Respir. Crit. Care Med. 5, 76–88 (1983).
Smythe, W. et al. A semimechanistic pharmacokinetic-enzyme turnover model for rifampin autoinduction in adult tuberculosis patients. Antimicrob. Agents Chemother. 56, 2091–2098 (2012).
Wagner, C. C. & Langer, O. Approaches using molecular imaging technology—use of PET in clinical microdose studies. Adv. Drug Deliv. Rev. 63, 539–546 (2011).
Svensson, R. J. et al. A population pharmacokinetic model incorporating saturable pharmacokinetics and autoinduction for high rifampicin doses. Clin. Pharmacol. Ther. 103, 674–683 (2018).
Acocella, G., Bonollo, L., Mainardi, M., Margaroli, P. & Tenconi, L. Serum and urine concentrations of rifampicin administered by intravenous infusion in man. Arzneimittelforschung 27, 1221–1226 (1977).
Nitti, V., Virgilio, R., Patricolo, M. & Iuliano, A. Pharmacokinetic study of intravenous rifampicin. Chemotherapy 23, 1–6 (1977).
Loos, U. et al. Pharmacokinetics of oral and intravenous rifampicin during chronic administration. Klin. Wochenschr. 63, 1205–1211 (1985).
Boeree, M. J. et al. A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 191, 1058–1065 (2015).
Gumbo, T., Pasipanodya, J. G., Romero, K., Hanna, D. & Nuermberger, E. Forecasting accuracy of the hollow fiber model of tuberculosis for clinical therapeutic outcomes. Clin. Infect. Dis. 61 (Suppl. 1), S25–S31 (2015).
Acknowledgements
We thank all the patients who participated in the study. Additionally, we thank the Maryland Department of Health and Mental Hygiene for recruiting patients with TB; R. Abdallah, C. Voicu, J. Sanchez-Bautista, S. Frey and L. Shinehouse (Johns Hopkins Hospitals) for coordinating the human imaging studies; and M. Klunk for helping with the animal experiments. This work was funded by the US National Institutes of Health (grant R01-HL131829), Director’s Transformative Research Award (grant R01-EB020539) and R56-AI145435 to S.K.J.
Author information
Authors and Affiliations
Contributions
A.A.O. and S.K.J. conceptualized and designed the study. H.W., J.V.S.G. and V.D.I. developed the pharmacokinetic models and analysis. G.M., S.S. and T.G. developed the hollow-fiber system experiments and analysis. D.P.H., W.B.M. and R.F.D. synthesized [11C]rifampin. A.A.O., C.A.R.-B., M.E.U. and L.P. performed the rabbit experiments. A.A.O., A.C., E.W.T., M.R.S. and S.K.J. recruited patients with TB and obtained their consent. A.A.O. and C.A.R.-B. analyzed the imaging data. E.F.C. developed algorithms to visualize PET data as heat map overlays. M.A.L. and S.P.R. designed the imaging protocols and provided technical expertise on image analysis. C.A.P. performed the mass spectrometry analysis. A.A.O., H.W., G.M., V.D.I. and S.K.J. analyzed the data and performed statistical analysis. A.A.O. and S.K.J. wrote the initial draft, and all coauthors edited the manuscript. S.K.J. provided funding and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Alison Farrell was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Study outline and patient characteristics.
(a) Patients with pulmonary TB receiving a rifampin-based TB treatment were enrolled and 11C-rifampin PET/CT performed within 6-weeks of treatment initiation. A subset of patients (n = 2) was also imaged at least 20 weeks after starting treatment. (b) All patients were HIV negative and were receiving an oral regimen of isoniazid, rifampin, pyrazinamide, and ethambutol at the time of imaging, except for patient 5 that received moxifloxacin instead of ethambutol. Patient 7 was excluded from the study due to significant motion artifact during the 11C-rifampin PET/CT. Gender: male (M) and female (F).
Extended Data Fig. 2 Heterogeneous pulmonary lesions in different lung regions of TB patients.
(a) Transverse CT sections from the TB patients demonstrate the simultaneous presence of multiple pulmonary lesions. (b) TB lesions and cavities with different sizes (volumes) were noted in all lung regions, although there was a preference for the upper lung lobes. Data from all 12 patients is shown. (c and d) Using the CT as a reference, the volumes of interest (VOIs) were drawn and applied to the PET data. VOIs for unaffected lung were placed at the same location in the contralateral unaffected lung.
Extended Data Fig. 3 11C-Rifampin measurements in the liver and brain of TB patients.
(a) Time-activity curve of 11C-rifampin measured in the liver of patients. (b) AUC liver to plasma ratios (c) Time-activity curve of 11C-rifampin measured in the brain of patients. (d) AUC brain to plasma ratios. The red dots represent individual measurements [n = 12 VOIs (measured at 10 time-points; total 120 VOIs per organ) corresponding to the 12 patients], with the black line and grey region representing the median and interquartile range, respectively. Tissue to plasma AUCs are represented as median and interquartile range.
Extended Data Fig. 4 Intrasubject heterogeneity of 11C-rifampin exposure.
11C-Rifampin area under the concentration-time curve (AUC) obtained by integrating the area under the dynamic 11C-rifampin PET time-activity curves (middle panels) as well as the AUC ratio (tissue to plasma) (right panels) from selected patients, are shown as heatmap overlays on the CT (left panels). The dotted white lines outline the lesion/cavity. Tissue density [X-ray attenuation value (Hounsfield Unit)] obtained via CT was used to correct the corresponding PET data to represent 11C-rifampin concentrations per mass of tissue.
Extended Data Fig. 5 The rabbit model of cavitary TB mimics human disease.
(a) Scheme describing the experiments performed in the rabbit model. (b) After aerosol infection with M. tuberculosis, the rabbits developed a heterogeneous pulmonary disease similar to humans. Transverse CT sections from rabbits at week 18 post-infection are shown. (c) Noninvasive longitudinal monitoring of disease progression using CT. Cavities/lesions are indicated with red arrows. (d) Histopathological analysis of infected-rabbit tissues demonstrates acid-fast bacilli (AFB) in the necrotic areas of the granuloma and cavity wall. Surrounding fibrosis (blue regions) can be noted with Mason’s trichrome staining. Histological analyses were repeated independently four times with similar results.
Extended Data Fig. 6 11C-Rifampin PET in rabbits.
Time-activity curves for individual lesions (a) cavity walls, (b) TB lesions and (c) unaffected lung regions from M. tuberculosis-infected rabbits.
Extended Data Fig. 7 Direct tissue measurements of rifampin (mass spectrometry) in rabbits.
(a) Gross pathology demonstrating the M. tuberculosis-infected lungs and the (b) areas selected for quantification of rifampin by mass spectrometry. U, unaffected lung (white); L, lesion (blue); C, cavity wall (red). (c) Tissue concentrations of rifampin and its metabolite 25-desacetyl rifampin. ND, not detectable. The lung tissues from each rabbit were processed independently (n = 5 animals).
Extended Data Fig. 8 Rifampin concentrations in rabbit tissues before and after treatment.
All rabbits received five oral doses of rifampin prior to PET imaging to achieve steady state. No differences were identified in (a) 11C-rifampin PET AUC tissue/plasma ratios (n = 11 cavity wall lesions, 13 TB lesions, and 21 unaffected lung regions are shown), (b) absolute rifampin concentrations measured by mass spectrometry (n = 10 cavity wall lesions, 19 TB lesions, 20 unaffected lung regions, caseum from 10 lesions, and five plasma samples are shown), or (c) the tissue/plasma ratios for TB lesions using mass spectrometry quantification in animals before or after treatment (n = 10 cavity wall lesions, 19 TB lesions, 20 unaffected lung regions and caseum from 10 lesion are shown). Data represented as median ± interquartile range. Statistical comparisons performed using a one-tailed Mann-Whitney U test.
Extended Data Fig. 9 Pharmacokinetic lung-biodistribution model.
(a) Model schematic. Maximal elimination rate (Vmax), rifampin concentration at which the elimination is half-maximal (Km) and volume of distribution (Vc) of the central compartment of therapeutic dose, clearance (CLmic) and volume of distribution (Vc,mic) of the central compartment of the 11C-rifampin dose, partition coefficient for left ventricle (PCLV), unaffected lung (PCUL), pulmonary lesion (PCPL), cavity wall (PCCW), equilibration rate constant (Keq) between lung and venous plasma, equilibration rate constant (Keq-lv) between left ventricle and venous plasma, transit rate constant (Ktr), maximal increase in the enzyme production rate (Smax), rifampin concentration at which half the Smax is reached (SC50), and rate constant for the first-order degradation of the enzyme pool (kenz). (b) Individual fittings of observed (red dots) and model-predicted 11C-rifampin concentrations (black lines) in plasma and pulmonary tissues from all patients. Patient 7 was excluded from the study due to significant motion artifact during the 11C-rifampin PET/CT.
Extended Data Fig. 10 Pharmacokinetic model with observed and previously published data.
(a) Observed and model-predicted 11C-rifampin exposure AUC in plasma, unaffected lung, TB lesions and cavity walls of all patients. (b) External data digitized from these studies on TB patients receiving varying doses of intravenous rifampin were used to enrich the dataset. (c, d) Model-predicted rifampin plasma AUC24h and Cmax at steady state compared to the data previously reported by Boree et al. (Am J Respir Crit Care Med. 2015).
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Supplementary Tables 1–6.
Rights and permissions
About this article
Cite this article
Ordonez, A.A., Wang, H., Magombedze, G. et al. Dynamic imaging in patients with tuberculosis reveals heterogeneous drug exposures in pulmonary lesions. Nat Med 26, 529–534 (2020). https://doi.org/10.1038/s41591-020-0770-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-020-0770-2
This article is cited by
-
Dynamic 18F-Pretomanid PET imaging in animal models of TB meningitis and human studies
Nature Communications (2022)
-
Microfluidic dose–response platform to track the dynamics of drug response in single mycobacterial cells
Scientific Reports (2022)
-
Microdosing as a Potential Tool to Enhance Clinical Development of Novel Antibiotics: A Tissue and Plasma PK Feasibility Study with Ciprofloxacin
Clinical Pharmacokinetics (2022)
-
124I-Iodo-DPA-713 Positron Emission Tomography in a Hamster Model of SARS-CoV-2 Infection
Molecular Imaging and Biology (2022)
-
Genomic analyses of Mycobacterium tuberculosis from human lung resections reveal a high frequency of polyclonal infections
Nature Communications (2021)