Abstract
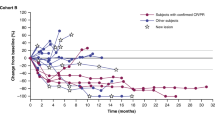
Oncogene-targeted therapy with B-Raf proto-oncogene (BRAF) and mitogen-activated protein kinase kinase (MEK) inhibitors induces a high initial response rate in patients with BRAF V600-mutated melanoma, with a median duration of response of approximately 1 year1,2,3. Immunotherapy with antibodies to programmed death 1 (PD-1) produces lower response rates but with long response duration. Preclinical models suggest that combining BRAF and MEK inhibitors with PD-1 blockade therapy improves antitumor activity4,5,6, which may provide additional treatment options for patients unlikely to have long-lasting responses to either mode of therapy alone. We enrolled 15 patients with BRAF V600-mutated metastatic melanoma in a first-in-human clinical trial of dabrafenib, trametinib and pembrolizumab (NCT02130466). Eleven patients (73%) experienced grade 3/4 treatment-related adverse events, the most common being elevation of liver function tests and pyrexia, most of which resolved with drug interruption or discontinuation of either the anti-PD-1 antibody or the targeted therapy combination. Eleven patients (73%; 95% confidence interval = 45–92%) had an objective response, and six (40%; 95% confidence interval = 16–68%) continued with a response at a median follow-up of 27 months (range = 10.3–38.4+ months) for all patients. This study suggests that this triple-combined therapy may benefit a subset of patients with BRAF V600-mutated metastatic melanoma by increasing the frequency of long-lasting antitumor responses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
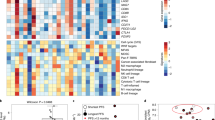
To protect the privacy and confidentiality of the 22 patients in this small study, RNA-Seq and WES data supporting the GEP and TMB analyses have not been made publicly available in a repository and are instead provided in the Supplementary Material linked to this article. Patient BRAF mutation status, GEP and TMB scores, and available RNA-Seq and WES data are listed in Supplementary Table 10, and anonymized patient-level somatic mutation data are provided in Supplementary Table 11. Requests for access to patient-level clinical data from the KEYNOTE trial in this study can be submitted through the EngageZone site (http://engagezone.msd.com/ds_documentation.php) or via email (dataaccess@merck.com) per Merck’s data-sharing policy. Research proposals involving predictive biomarker data should include statistical analysis plans that describe a prespecified hypothesis and accompanying statistical power calculation. Studies of de novo biomarker discovery must additionally include descriptions of independent training and validation sets.
Change history
02 July 2019
This sentence was originally included in the legend for Extended Data Fig. 1: "*Nominal time after first dose. N denotes number of patients with samples for pharmacokinetic analysis." The sentence was deleted as no asterisk appeared in that figure.
References
Larkin, J. et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 371, 1867–1876 (2014).
Long, G. V. et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 371, 1877–1888 (2014).
Robert, C. et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 372, 30–39 (2015).
Homet Moreno, B., Mok, S., Comin-Anduix, B., Hu-Lieskovan, S. & Ribas, A. Combined treatment with dabrafenib and trametinib with immune-stimulating antibodies for BRAF mutant melanoma. Oncoimmunology 5, e1052212 (2016).
Deken, M. A. et al. Targeting the MAPK and PI3K pathways in combination with PD1 blockade in melanoma. Oncoimmunology 5, e1238557 (2016).
Hu-Lieskovan, S. et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF V600E melanoma. Sci. Transl. Med. 7, 279ra41 (2015).
Akbani, R. et al. Genomic classification of cutaneous melanoma. Cell 161, 1681–1696 (2015).
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
Hauschild, A. et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380, 358–365 (2012).
Su, F. et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N. Engl. J. Med. 366, 207–215 (2012).
Ribas, A., Hodi, F. S., Callahan, M., Konto, C. & Wolchok, J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N. Engl. J. Med. 368, 1365–1366 (2013).
Vella, L. J. et al. MEK inhibition, alone or in combination with BRAF inhibition, affects multiple functions of isolated normal human lymphocytes and dendritic cells. Cancer Immunol. Res. 2, 351–360 (2014).
Long, G. V. et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J. Clin. Oncol. 29, 1239–1246 (2011).
Shi, H. et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 4, 80–93 (2014).
Van Allen, E. M. et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 4, 94–109 (2014).
Ayers, M. et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 127, 2930–2940 (2017).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Rizvi, N. A. et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
Hugo, W. et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell 165, 35–44 (2016).
Daud, A. I. et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J. Clin. Oncol. 34, 4102–4109 (2016).
Wilmott, J. S. et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin. Cancer Res. 18, 1386–1394 (2012).
Frederick, D. T. et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin. Cancer Res. 19, 1225–1231 (2013).
Chen, P. L. et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 6, 827–837 (2016).
Vilain, R. E. et al. Dynamic changes in PD-L1 expression and immune infiltrates early during treatment predict response to PD-1 blockade in melanoma. Clin. Cancer Res. 23, 5024–5033 (2017).
K EYTRUDA ® (Pembrolizumab) for Injection, for Intravenous Use (Merck Sharp & Dohme Corp., 2019).
T afinlar 50 mg Hard Capsules (Novartis Europharm, 2015).
M EKINIST (Trametinib) Tablets, for Oral Use (GlaxoSmithKline, 2018).
Schadendorf, D. et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur. J. Cancer 82, 45–55 (2017).
Postow, M. A. et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372, 2006–2017 (2015).
Wolchok, J. D. et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 377, 1345–1356 (2017).
Schachter, J. et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 390, 1853–1862 (2017).
Ribas, A. et al. Phase I study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and/or MEK (trametinib) inhibitors in advanced melanoma. J. Clin. Oncol. 33, 3003 (2015).
Lyle, S. et al. Human hair follicle bulge cells are biochemically distinct and possess an epithelial stem cell phenotype. J. Investig. Dermatol. Symp. Proc. 4, 296–301 (1999).
Nuckols, J. D., Shea, C. R., Horenstein, M. G., Burchette, J. L. & Prieto, V. G. Quantitation of intraepidermal T-cell subsets in formalin-fixed, paraffin-embedded tissue helps in the diagnosis of mycosis fungoides. J. Cutan. Pathol. 26, 169–175 (1999).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Grasso, C. S. et al. Genetic mechanisms of immune evasion in colorectal cancer. Cancer Discov. 8, 730–749 (2018).
Grasso, C. S. et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 487, 239–243 (2012).
Acknowledgements
Funding for this research was provided by Merck Sharpe & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. The authors thank the patients and their families, all primary investigators and site personnel for participation in the study. The authors thank S. Ebbinghaus and S. Diede for study design and oversight, M. Bucci, J. Siegel and N. Cote for data acquisition, A. L. Webber for biomarker analysis, M. Nebozhyn for GEP analysis, R. Cristescu for WES analysis; Y. Cui for CD8 analysis, and R. Mogg for biomarker analysis. Editorial assistance was provided by D. Mitra of the ApotheCom pembrolizumab team. This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. A.R., J.T. and C.S.G. were funded by NIH grants R35 CA197633 and P01 CA168585, and by the Parker Institute for Cancer Immunotherapy.
Author information
Authors and Affiliations
Contributions
A.R., O.H., G.V.L., B.M. and B.H.M. conceived, designed or planned the study. V.A., B.H.M., M.S.C., O.H., F.S.H., G.V.L., N.I. and A.R. acquired the data. Q.Z., B.H.M., F.S.H., G.V.L., B.M., N.I. and A.R. analyzed the data. V.A., B.H.M., M.S.C., F.S.H., G.V.L., B.M., N.I. and A.R. interpreted the results. J.T. and C.S.G., together with R.G., analyzed the genomic data. A.R. wrote the first draft of the manuscript, with contributions from Q.Z., V.A., B.H.M., O.H. and N.I. M.S.C., O.H., F.S.H., G.V.L., B.M., N.I. and A.R. critically reviewed or revised the manuscript for important intellectual content. All authors reviewed the interim drafts and final version of the manuscript, and agreed with its content and submission. All authors had access to all of the relevant study data and related analyses, and vouch for the completeness and accuracy of the presented data. All authors agree to be accountable for all aspects of the work and will ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
A.R. received personal fees for consulting from Amgen, Bristol-Myers Squibb, Chugai, Genentech–Roche, Merck–MSD and Novartis, and is on the scientific advisory board of, and holds stock in, Advaxis, Apricity, Arcus, Bioncotech, Compugen, CytomX, Five Prime, FLX Bio, ImaginAb, Isoplexis, Kite-Gilead, Merus, Rgenix, Lutris, PACT Pharma and Tango Therapeutics. V.A. received personal fees and non-financial support from Bristol-Myers Squibb and Merck Sharpe & Dohme, and personal fees only from Merck Serono, Novartis and Pierre Fabre. W.H.M. received personal fees from Bristol-Myers Squibb, Novartis, GlaxoSmithKline, Amgen and Roche. M.S.C. received personal fees from Merck Sharpe & Dohme, Novartis, Bristol-Myers Squibb and Pierre Fabre. R.F. received personal fees from Merck Sharpe & Dohme. G.V.L. received personal fees from Aduro, Amgen, Array, Bristol-Myers Squibb, Merck Sharpe & Dohme, Novartis, Oncosec and Pierre Fabre. F.S.H. reports receiving personal fees from Merck, Bristol-Myers Squibb, EMD Serono, Celldex, Sanofi, and Novartis; other from Merck to his institution and he has a patent on MICA related disorders for which he receives royalties. B.M. reports employment and stock options at Novartis and stock options at GlaxoSmithKline. B.H.M., Q.Z., R.G. and N.I. report employment at Merck Sharp & Dohme. N.I. also holds stocks in Merck & Co. and GlaxoSmithKline. O.H. reports personal fees from Merck, during the conduct of the study; personal fees from Amgen, Novartis, Roche, Bristol-Myers Squibb, Genenetch, and contracted institutional support from AstraZeneca, Bristol-Myers Squibb, Celldex, Genentech, Immunocore, Incyte, Merck, MerckSerono, MedImmune, Novartis, Pfizer, Rinat and Roche. All other authors have no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Pharmacokinetic concentration-time profiles of pembrolizumab, dabrafenib and trametinib.
Pharmacokinetic concentration-time profiles of (a) pembrolizumab, (b) dabrafenib and (c) trametinib following administration of 2 mg/kg pembrolizumab intravenously administered together with multiple oral administrations dabrafenib 150 mg twice daily and trametinib 2 mg daily. Individual concentrations/profiles are presented as colored lines for pembrolizumab and open circles for dabrafenib and trametinib. Arithmetic mean concentration-time profiles (±standard error) are presented as dotted black bold lines.
Extended Data Fig. 2 Dosing of pembrolizumab in part 2: dose expansion.
Pembrolizumab 2 mg/kg Q3W, trametinib 2 mg QD, and dabrafenib 150 mg BID in the dose expansion phase. BID, twice daily; Q3W, every 3 weeks; QD, once daily.
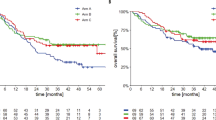
Extended Data Fig. 3 Kaplan-Meier estimates of progression-free survival and overall survival.
(a) Progression-free survival and (b) overall survival.
Supplementary information
Supplementary Information
Supplementary Tables 1–10 and Supplementary Protocol 1
Supplementary Table 11
Anonymized somatic mutation data of patients
Rights and permissions
About this article
Cite this article
Ribas, A., Lawrence, D., Atkinson, V. et al. Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat Med 25, 936–940 (2019). https://doi.org/10.1038/s41591-019-0476-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-019-0476-5
This article is cited by
-
New clinical trial design in precision medicine: discovery, development and direction
Signal Transduction and Targeted Therapy (2024)
-
Unique vulnerability of RAC1-mutant melanoma to combined inhibition of CDK9 and immune checkpoints
Oncogene (2024)
-
Targeting MEK/COX-2 axis improve immunotherapy efficacy in dMMR colorectal cancer with PIK3CA overexpression
Cellular Oncology (2024)
-
Molecular characterization of Chinese patients with small bowel adenocarcinoma
Clinical and Translational Oncology (2024)
-
System analysis based on the pyroptosis-related genes identifes GSDMD as a novel therapy target for skin cutaneous melanoma
Journal of Translational Medicine (2023)