Abstract
Melanoma treatment has progressed in the past decade with the development and approval of immune checkpoint inhibitors targeting programmed death 1 (PD-1) or its ligand (PD-L1) and cytotoxic T lymphocyte-associated antigen 4, as well as small molecule inhibitors of BRAF and/or MEK for the subgroup of patients with BRAFV600 mutations1,2,3,4,5,6,7,8,9. BRAF/MEK-targeted therapies have effects on the tumor microenvironment that support their combination with PD-1/PD-L1 inhibitors10,11,12,13,14,15,16,17,18,19,20. This phase Ib study (ClinicalTrials.gov, number NCT01656642) evaluated the safety and anti-tumor activity of combining atezolizumab (anti-PD-L1) with vemurafenib (BRAF inhibitor), or cobimetinib (MEK inhibitor) + vemurafenib, in patients with BRAFV600-mutated metastatic melanoma. Triple combination therapy with atezolizumab + cobimetinib + vemurafenib, after a 28-d run-in period with cobimetinib + vemurafenib, had substantial but manageable toxicity. Exploratory biomarker data show that the cobimetinib + vemurafenib run-in was associated with an increase in proliferating CD4+ T-helper cells but not with an increase in T-regulatory cells, as observed in the vemurafenib-only run-in period. The confirmed objective response rate was 71.8% (95% confidence interval 55.1–85.0). The estimated median duration of response was 17.4 months (95% confidence interval 10.6–25.3) with ongoing response in 39.3% of patients after 29.9 months of follow-up. Further investigation in a phase III trial is underway.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the article and its Supplementary information, or available from the corresponding author upon reasonable request.
References
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
Hauschild, A. et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380, 358–365 (2012).
Larkin, J. et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 371, 1867–1876 (2014).
Robert, C. et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 372, 30–39 (2015).
Long, G. V. et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 386, 444–451 (2015).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Robert, C. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372, 2521–2532 (2015).
Weber, J. S. et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 16, 375–384 (2015).
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015).
Sumimoto, H., Imabayashi, F., Iwata, T. & Kawakami, Y. The BRAF–MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J. Exp. Med. 203, 1651–1656 (2006).
Khalili, J. S. et al. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 melanoma. Clin. Cancer Res. 18, 5329–5340 (2012).
Frederick, D. T. et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin. Cancer Res. 19, 1225–1231 (2013).
Wilmott, J. S. et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin. Cancer Res. 18, 1386–1394 (2012).
Schilling, B. & Paschen, A. Immunological consequences of selective BRAF inhibitors in malignant melanoma: neutralization of myeloid-derived suppressor cells. Oncoimmunology 2, e25218 (2013).
Long, G. V. et al. Effects of BRAF inhibitors on human melanoma tissue before treatment, early during treatment, and on progression. Pigment Cell Melanoma Res. 26, 499–508 (2013).
Boni, A. et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 70, 5213–5219 (2010).
Kono, M. et al. Role of the mitogen-activated protein kinase signaling pathway in the regulation of human melanocytic antigen expression. Mol. Cancer Res. 4, 779–792 (2006).
Sapkota, B., Hill, C. E. & Pollack, B. P. Vemurafenib enhances MHC induction in BRAF(V600E) homozygous melanoma cells. Oncoimmunology 2, e22890 (2013).
Vella, L. J. et al. MEK inhibition, alone or in combination with BRAF inhibition, affects multiple functions of isolated normal human lymphocytes and dendritic cells. Cancer Immunol. Res. 2, 351–360 (2014).
Cooper, Z. A. et al. BRAF inhibition is associated with increased clonality in tumor-infiltrating lymphocytes. Oncoimmunology 2, e26615 (2013).
Ascierto, P. A. et al. The role of BRAF V600 mutation in melanoma. J. Transl. Med. 10, 85 (2012).
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002).
Curtin, J. A. et al. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med. 353, 2135–2147 (2005).
Wan, P. T. et al. Mechanism of activation of the RAF–ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116, 855–867 (2004).
Ascierto, P. et al. Cobimetinib combined with vemurafenib in advanced BRAF V600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 17, 1248–1260 (2016).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Rosenberg, J. E. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387, 1909–1920 (2016).
Garon, E. B. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 372, 2018–2028 (2015).
Ebert, P. J. R. et al. MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity 44, 609–621 (2016).
Cooper, Z. A. et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol. Res. 2, 643–654 (2014).
Hu-Lieskovan, S. et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci. Transl. Med. 7, 279ra241–279ra241 (2015).
Hamid, O. et al. Preliminary clinical safety, tolerability and activity of atezolizumab (anti-PDL1) combined with vemurafenib in BRAFV600 metastatic melanoma. Pigment Cell Melanoma Res. 28, 778 (2015).
Sullivan, R. et al. Preliminary clinical safety, tolerability and activity results from a Phase Ib study of atezolizumab (antiPDL1) combined with vemurafenib in BRAFV600 mutant metastatic melanoma. J. Transl. Med. 14, 9–10 (2016).
Rapisuwon, S. et al. Analysis of the kinetics and effects of vemurafenib (V) plus cobimetinib (C) on intratumoral and host immunity in patients (pts) with BRAFV600 mutant melanoma (BRAFmM): implications for combination with immunotherapy. J. Clin. Oncol. 36, 9559–9559 (2018).
Ribas, A. et al. KEYNOTE-022 update: phase 1 study of first-line pembrolizumab (pembro) plus dabrafenib (D) and trametinib (T) for BRAF-mutant advanced melanoma. Ann. Oncol. 28, v428–v448 (2017).
Long, G. V. et al. Impact of baseline serum lactate dehydrogenase concentration on the efficacy of pembrolizumab and ipilimumab in patients with advanced melanoma: data from KEYNOTE-006. Eur. J. Cancer 72, S122–S123 (2017).
Long, G. V. et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 17, 1743–1754 (2016).
Daud, A. I. et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J. Clin. Oncol. 34, 4102–4109 (2016).
Wongchenko, M. J. et al. Gene expression profiling in BRAF-mutated melanoma reveals patient subgroups with poor outcomes to vemurafenib that may be overcome by cobimetinib plus vemurafenib. Clin. Cancer Res. 23, 5238–5245 (2017).
Acknowledgements
Research support was provided by S. Lecagoonporn (MD Anderson Cancer Center, Houston, TX, USA). Medical writing support was provided by M. Sweetlove and J. Sah (ApotheCom, San Francisco, CA, USA) and was funded by F. Hoffmann-La Roche Ltd.
Author information
Authors and Affiliations
Contributions
R.J.S., O.H., M.B., E.C. and P.H. designed the study. R.S., O.H., P.H., R.G., J.R.I., M.R.P., F.S.H., K.D.L., H.A.T., G.H., Y.Y. and M.J.W. provided study material, patients and expert guidance. R.J.S., O.H., M.J.W., Y.Y., Y.C., L.R., M.B., E.C. and P.H. contributed to data analysis and interpretation. All authors reviewed, revised and provided input on the manuscript.
Corresponding author
Ethics declarations
Competing interests
This study was funded by F. Hoffman-La Roche Ltd. R.J.S. reports personal fees from Amgen, Merck, Genentech, Array and Novartis; research grants from Amgen and Merck; and clinical trial support from Merck, Genentech and Novartis during the conduct of the study; and by personal fees from Compugen, Replimune and Syndax outside the submitted work. O.H. reports advisory board fees from Roche and speaker bureau fees from Genentech during the conduct of the study; advisory board fees from Amgen, Novartis and Bristol-Myers Squibb; speaker bureau fees from Amgen, Bristol-Myers Squibb, Novartis and Array Biopharma; and research support paid to institution from AstraZeneca, Bristol-Myers Squibb, Celldex, Genentech, Immunocore, Merck Serono, MedImmune, Novartis, Pfizer, Rinat and Roche outside the submitted work. R.G. reports personal fees and grants from Roche/Genentech during the conduct of the study, personal fees and grants from Bristol-Myers Squibb and Novartis; and grants from Merck and Array Biopharma outside the submitted work. J.R.I. reports consultancy fees from BioMed Valley and Armo Biosciences and employment with Janssen Pharmaceuticals outside the submitted work. M.R.P. reports no competing interests. F.S.H. reports clinical trial support paid to institution from Genentech during the conduct of the study; consultancy fees from Genentech, Merck, Novartis, Bristol-Myers Squibb, Aduro, Partner Therapeutics, EMD Serono, Sanofi and Celldex; advisory board fees from Apricity; and research grants paid to institution from Bristol-Myers Squibb outside the submitted work; F.S.H. also reports an issued patent for Therapeutic Peptides and pending patents for MICA Related Disorders and Angiopoietin-2 as Therapeutic Target for Cancer. K.D.L. reports grants from Roche/Genentech during the conduct of the study and consultancy fees from Roche/Genentech outside the submitted work. H.A.T. reports grants and personal fees from Roche/Genentech during the conduct of the study; and grants and personal fees from Bristol-Myers Squibb, Merck and Novartis outside the submitted work. G.H. reports employment with Roche. M.J.W. reports employment and stock ownership with Genentech/Roche and stock ownership with ARIAD Pharmaceuticals. Y.C. reports employment with Genentech/Roche. L.R. reports employment with Genentech/Roche. M.B. reports employment and stock ownership with Genentech/Roche. Y.Y. reports employment with Genentech/Roche and stock ownership with Genentech/Roche. E.C. reports employment and stock ownership with Genentech/Roche. P.H. reports consultancy and advisory fees from Dragonfly, GlaxoSmithKline, Immatics and Sanofi outside the submitted work.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
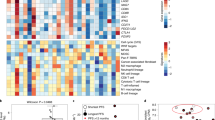
Extended Data Fig. 1 Study design and patients.
a, Patients received at least one dose of atezolizumab during the combination treatment period (safety population). b, Patients with progression after prior checkpoint inhibitor therapy (mandatory serial biopsy). c, Checkpoint inhibitor-naive patients (mandatory serial biopsy). d, checkpoint inhibitor-naive patients (no mandatory serial biopsy).
Extended Data Fig. 2 Longitudinal change in tumor burden.
a,b, Longitudinal change in tumor burden in: patients treated with atezolizumab + vemurafenib (n = 17) (a) and patients treated with atezolizumab + cobimetinib + vemurafenib (n = 39) (b). PD, progressive disease.
Extended Data Fig. 3 CD8+ T cells in the tumor center.
Representative images of CD8+ T cells in the tumor center before and after cobimetinib + vemurafenib treatment in 3 of 6 patients treated in the dose-escalation study phase. Numbers in each panel of a represent percentage of CD8+ cells in the tumor center. CR, complete response; SD, stable disease.
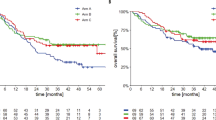
Extended Data Fig. 4 Survival by PD-L1 expression.
a–d, Kaplan–Meier curves of: PFS according to PD-L1 expression in patients treated with atezolizumab + vemurafenib (a); OS according to PD-L1 expression in patients treated with atezolizumab + vemurafenib (b); PFS according to PD-L1 expression in patients treated with atezolizumab + cobimetinib + vemurafenib (c); and OS according to PD-L1 expression in patients treated with atezolizumab + cobimetinib + vemurafenib (d).
Extended Data Fig. 5 PFS by pre-treatment CD8+ T cell infiltration status.
a,b, PFS according to pre-treatment CD8+ T cell infiltration by CD8+ immunohistochemistry with: atezolizumab + vemurafenib (a) and atezolizumab + cobimetinib + vemurafenib (b). c,d, PFS according to pre-treatment CD8+ T cell infiltration by Teff signature with: atezolizumab + vemurafenib (c) and atezolizumab + cobimetinib + vemurafenib (d).
Extended Data Fig. 6 Gating strategies for FACS.
a,b, Gating strategies for FASC panels for: T cell activation/proliferation (a) and regulatory T cells (b).
Supplementary information
Supplementary Information
Supplementary Tables 1–6.
Rights and permissions
About this article
Cite this article
Sullivan, R.J., Hamid, O., Gonzalez, R. et al. Atezolizumab plus cobimetinib and vemurafenib in BRAF-mutated melanoma patients. Nat Med 25, 929–935 (2019). https://doi.org/10.1038/s41591-019-0474-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-019-0474-7
This article is cited by
-
The role of CRAF in cancer progression: from molecular mechanisms to precision therapies
Nature Reviews Cancer (2024)
-
Targeting MEK/COX-2 axis improve immunotherapy efficacy in dMMR colorectal cancer with PIK3CA overexpression
Cellular Oncology (2024)
-
Macrophage’s role in solid tumors: two edges of a sword
Cancer Cell International (2023)
-
The role of mitochondria in the resistance of melanoma to PD-1 inhibitors
Journal of Translational Medicine (2023)
-
The role of triple therapy and therapy sequence in treatment of BRAF-mutant metastatic melanoma. Response to overall survival with first-line atezolizumab in combination with vemurafenib and cobimetinib in BRAFV600 mutation-positive advanced melanoma (IMspire150): second interim analysis of a multicentre, randomised, phase 3 study
Journal of Translational Medicine (2023)