Abstract
Anti-CD19 chimeric antigen receptor (CAR) T cell therapies can cause severe cytokine-release syndrome (CRS) and neurotoxicity, impeding their therapeutic application. Here we generated a new anti-CD19 CAR molecule (CD19-BBz(86)) derived from the CD19-BBz prototype bearing co-stimulatory 4-1BB and CD3ζ domains. We found that CD19-BBz(86) CAR T cells produced lower levels of cytokines, expressed higher levels of antiapoptotic molecules and proliferated more slowly than the prototype CD19-BBz CAR T cells, although they retained potent cytolytic activity. We performed a phase 1 trial of CD19-BBz(86) CAR T cell therapy in patients with B cell lymphoma (ClinicalTrials.gov identifier NCT02842138). Complete remission occurred in 6 of 11 patients (54.5%) who each received a dose of 2 × 108–4 × 108 CD19-BBz(86) CAR T cells. Notably, no neurological toxicity or CRS (greater than grade 1) occurred in any of the 25 patients treated. No significant elevation in serum cytokine levels after CAR T cell infusion was detected in the patients treated, including in those who achieved complete remission. CD19-BBz(86) CAR T cells persistently proliferated and differentiated into memory cells in vivo. Thus, therapy with the new CD19-BBz(86) CAR T cells produces a potent and durable antilymphoma response without causing neurotoxicity or severe CRS, representing a safe and potent anti-CD19 CAR T cell therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Imai, C. et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 18, 676–684 (2004).
Campana, D., Schwarz, H. & Imai, C. 4-1BB chimeric antigen receptors. Cancer J. 20, 134–140 (2014).
Milone, M. C. et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 17, 1453–1464 (2009).
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C. H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Maude, S. L. P. et al. Efficacy and safety of CTL019 in the first US phase II multicenter trial in pediatric relapsed/refractory acute lymphoblastic leukemia: results of an interim analysis. Blood 128, 2801 (2016).
Schuster, S. J. et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 377, 2545–2554 (2017).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019).
Eshhar, Z., Waks, T., Gross, G. & Schindler, D. G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl Acad. Sci. USA 90, 720–724 (1993).
Brentjens, R. J. et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin. Cancer Res. 13, 5426–5435 (2007).
Kochenderfer, J. N. et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010).
Kalos, M. et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 3, 95ra73 (2011).
Kochenderfer, J. N. et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119, 2709–2720 (2012).
Brentjens, R. J. et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 5, 177ra138 (2013).
Lee, D. W. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195 (2014).
Turtle, C. J. et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 8, 355ra116 (2016).
Turtle, C. J. et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 126, 2123–2138 (2016).
Neelapu, S. S. et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat. Rev. Clin. Oncol. 15, 47–62 (2018).
Brudno, J. N. & Kochenderfer, J. N. Chimeric antigen receptor T-cell therapies for lymphoma. Nat. Rev. Clin. Oncol. 15, 31–46 (2018).
Kelley, L. A. & Sternberg, M. J. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4, 363–371 (2009).
Wang, X. et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 118, 1255–1263 (2011).
Giavridis, T. et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 24, 731–738 (2018).
Norelli, M. et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 24, 739–748 (2018).
Kochenderfer, J. N. et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 33, 540–549 (2015).
Porter, D. L. et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 7, 303ra139 (2015).
Teachey, D. T. et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 6, 664–679 (2016).
Brudno, J. N. & Kochenderfer, J. N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127, 3321–3330 (2016).
Gattinoni, L. et al. A human memory T cell subset with stem cell-like properties. Nat. Med. 17, 1290–1297 (2011).
Farber, D. L., Yudanin, N. A. & Restifo, N. P. Human memory T cells: generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 14, 24–35 (2014).
Wang, A. et al. The stoichiometric production of IL-2 and IFN-γ mRNA defines memory T cells that can self-renew after adoptive transfer in humans. Sci. Transl. Med. 4, 149ra120 (2012).
Hudecek, M. et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol. Res. 3, 125–135 (2015).
Alabanza, L. et al. Function of novel anti-CD19 chimeric antigen receptors with human variable regions is affected by hinge and transmembrane domains. Mol. Ther. 25, 2452–2465 (2017).
Waage, A., Brandtzaeg, P., Halstensen, A., Kierulf, P. & Espevik, T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J. Exp. Med. 169, 333–338 (1989).
Oliver, J. C. et al. Cytokine kinetics in an in vitro whole blood model following an endotoxin challenge. Lymphokine Cytokine Res. 12, 115–120 (1993).
Salter, A. I. et al. Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci. Signal. 11, eaat6753 (2018).
Marasco, W. A., Haseltine, W. A. & Chen, S. Y. Design, intracellular expression, and activity of a human anti-human immunodeficiency virus type 1 gp120 single-chain antibody. Proc. Natl Acad. Sci. USA 90, 7889–7893 (1993).
Shen, L., Evel-Kabler, K., Strube, R. & Chen, S. Y. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat. Biotechnol. 22, 1546–1553 (2004).
Song, X. T. et al. A20 is an antigen presentation attenuator, and its inhibition overcomes regulatory T cell-mediated suppression. Nat. Med. 14, 258–265 (2008).
Wang, D. et al. Efficacy of intracellular immune checkpoint-silenced DC vaccine. JCI Insight 3, e98368 (2018).
Jiang, X. X. et al. Control of B cell development by the histone H2A deubiquitinase MYSM1. Immunity 35, 883–896 (2011).
Acknowledgements
We would like to thank S. Yan, X. Wu, Y. Yan and members of the GMP-compliant CAR vector and CAR T cell manufacturing teams and the immunological monitoring team of Marino Biotechnology Corp. for their technical assistance. We would also like to thank Y. Wei and T. Liu for their valuable suggestions and help. This study was supported financially by the National Natural Science Foundation of China (No. 81600164, No. 81870154), Beijing Natural Science Foundation (No. 7172046), Beijing Municipal Administration of Hospitals Incubating Program (PX2017001), Capital’s Funds for Health Improvement and Research (No. 2018-1-2151), Beijing Municipal Administration of Hospitals’ Ascent Plan (No. DFL20151001), a generous donation from Y. L. Zhu and the Marino Biotechnology Corp.
Author information
Authors and Affiliations
Contributions
Z.Y., X.X., J. Zhu and S.-Y.C. designed the clinical trial; S.-Y.C., X.X. and X.F.H. designed the overall project. X.X., X.F.H., Y. Liu, X.K., X.G., H.L., T.Z., P.D., J. Zhang, Y.W., S.L., M.M., X.Y., L.F., S.W., S.L., H.S., G.W., S.-Y.C. and L.J. performed the experiments. Z.Y., J. Zhu, Y.S., N.D., Y. Lin, W.Z., Xiaopei Wang, N.L., M.T., Y.X., C.Z., W.L., L.D., S.G., L.P., Xuejuan Wang and N.Z. performed the clinical trial. S.-Y.C., X.X., X.F.H., Z.Y. and J. Zhu analyzed the results and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
X.X., Y. Liu, X.G., H.L., T.Z., P.D., J. Zhang, Y.W., S.L., M.M., X.Y., L.F., S.W. and H.S. are employees of Marino Biotechnology Corp., whose potential product was studied in this work. S.-Y.C. is a consultant of Marino Biotechnology Corp. and a recipient of a research contract with the corporation.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 In vitro and in vivo evaluation.
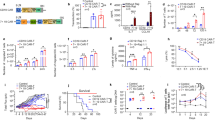
a, IL-6 levels in co-culture of monocytes and CD19-BBz-variant-transduced CAR T cells. Human T cells transduced with the indicated CD19-BBz variants were co-cultured with irradiated CD19-K562 cells in the presence of autologous monocytes (Mono) in a 24-well plate with or without a Corning Transwell (TW) to separate CAR T cells from monocytes. The culture medium was collected after 48 h of co-culture for analysis of IL-6 concentration by ELISA. Data are presented as the mean ± s.d. Experiments were repeated with four different donor-derived T cells (n = 4). A two-tailed, unpaired two-sample t test was used for statistical analysis. *P < 0.001, CD19-BBz(86) versus CD19-BBz(71). b, Proliferation of CD19-BBz-variant-transduced CAR T cells in co-culture with CD19-K562 cells. Human T cells were transduced with the indicated CD19-BBz variants and cultured for 1 week. tEGFR+ transduced CAR T cells were sorted by FACS and co-cultured with irradiated CD19-K562 cells. On the indicated days, T cells were counted, and the fold of T cell expansion is presented as the mean ± s.d. Experiments were repeated with three different donor-derived T cells (n = 3). c, [3H]thymidine incorporation assay to measure CD19-BBz-variant-transduced CAR T cell proliferation. Human T cells transduced with CD19-BBz variants were co-cultured with irradiated CD19-K562 cells in the presence of [3H]thymidine. Data are presented as the mean ± s.d. Experiments were repeated with four donor-derived T cells (n = 4). A two-tailed, unpaired two-sample t test was used for statistical analysis. *P < 0.001, CD19-BBz(86) versus CD19-BBz(71). d, Cytolytic activities of CD19-BBz-variant-transduced CAR T cells. CD19-BBz-variant-transduced CAR T cells were co-cultured with 51Cr-labeled CD19-K562 (left) or CD19+ Nalm-6 (right) cells in triplicate at the indicated E:T ratios. Cytotoxicity was measured by 51Cr release, and data are presented as the mean ± s.d. Experiments were repeated with three donor-derived T cells (n = 3). A two-tailed, unpaired two-sample t test was used for statistical analysis. NS, CD19-BBz(86) versus CD19-BBz(71). e, Annexin V expression in CD19-BBz-variant-transduced CAR T cells after co-culture with irradiated CD19-K562 cells or control K562 cells as detected by flow cytometry staining with anti-tEGFR and Annexin V. Data are presented as the mean ± s.d. Experiments were repeated with three donor-derived T cells (n = 3). A two-tailed, unpaired two-sample t test was used for statistical analysis. *P < 0.004, CD19-BBz(86) versus CD19-BBz(71). f, Serum mouse cytokine levels. SCID-beige mice were inoculated i.p. with 3 × 106 Raji cells followed by i.p. injection with 35 × 106 CD19-BBz(71) or CD19-BBz(86) CAR T cells or were mock treated. Sixty hours after CAR T cell injection, mice were bled and sera were isolated to determine the concentrations in serum of the indicated mouse cytokines by ELISA (n = 6 for the mock group, n = 12 for the CAR T cell groups). Data are presented as the mean ± s.d. A two-tailed, unpaired two-sample t test was used for statistical analysis. g, Absolute counts of intraperitoneal myeloid cell populations obtained by peritoneal lavage 60 h after injection of CAR T cells (i.p.). Data are presented as the mean ± s.d. (n = 4 for the mock group, n = 6 for the CAR T cell groups). A two-tailed, unpaired two-sample t test was used for statistical analysis. h, qRT–PCR analysis of mouse cytokine gene expression in intraperitoneal macrophages isolated from peritoneal lavage 60 h after injection of CAR T cells (i.p.). Data are presented as the mean ± s.d. (n = 4 for the mock group, n = 6 for the CAR T cell groups). A two-tailed, unpaired two-sample t test was used for statistical analysis. i, In vivo expansion of CAR T cells in tumor-bearing mice. Groups of NSG mice were inoculated intravenously (i.v.) with NALM-6 tumor cells followed by i.v. injection with CD19-BBz(71) or CD19-BBz(86) CAR T cells or mock T cells 4 d later. At days 7, 14 and 28 after CAR T cell injection, peripheral blood samples were collected for quantification of tEGFR+ CAR T cells in the blood. Data are presented as the mean ± s.d. (n = 4 for the mock group, n = 6 for the CAR T cell groups). A two-tailed, unpaired two-sample t test was used for statistical analysis.
Extended Data Fig. 2 Durable remission.
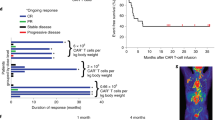
CT and PET–CT scans showing durable remission in patients BZ024 and BZ025 treated with CD19-BBz(86) CAR T cells. The arrow indicates sites of lymphoma. Scale bar, 10 cm.
Extended Data Fig. 3 Decrease in lymphocyte count after lymphodepletion chemotherapy in individual patients.
All patients were administrated 3-d lymphodepletion chemotherapy comprising fludarabine (25 mg m–2 on days 1–3) and cyclophosphamide (250 mg m–2 on days 1–3) before CAR T cell infusion. The time point of lymphodepletion was on the day of CAR T cell infusion (day 0) for all patients, except for patients BZ015 (day –1), BZ021 (day –2) and BZ026 (day –3). Each patient’s lymphocyte counts before and after lymphodepletion chemotherapy are connected by a line for pairwise comparison. The median blood lymphocyte count just before lymphodepletion chemotherapy was 1 × 109 cells L–1 (range of 0.49–1.73 × 109 cells L–1). The median lymphocyte count after chemotherapy on the day of CAR T cell infusion was 0.14 × 109 cells L–1 (range of 0.02–0.61 × 109 cells L–1).
Extended Data Fig. 4 Changes in blood IgA levels.
Changes in IgA levels were assessed after CD19-BBz(86) CAR T cell therapy in individual patients.
Extended Data Fig. 5 Changes in blood IgG levels.
Changes in IgG levels were assessed after CD19-BBz(86) CAR T cell therapy in individual patients.
Extended Data Fig. 6 Changes in blood IgM levels.
Changes in IgM levels were assessed after CD19-BBz(86) CAR T cell therapy in individual patients.
Extended Data Fig. 7 In vivo expansion and memory generation.
a, Flow cytometry analysis showing in vivo expansion of tEGFR+ CD19-BBz(86) CAR T cells in the peripheral blood of representative patients who achieved complete remission (patient BZ021) or had progressive disease (patient BZ013). b,c. qPCR showing in vivo CAR T cell expansion and persistence in the blood of patients who achieved complete or partial remission (patients BZ015, BZ016, BZ019, BZ020, BZ021, BZ024 and BZ025) (b) or had progressive disease (patients BZ017, BZ022 and BZ023) (c). d, Memory phenotype (CD45RA–CCR7+) of in vivo-expanded tEGFR+CD3+ CD19-BBz(86) CAR T cells and tEGFR–CD3+ normal T cells from six patients who had progressive disease (BZ013) or achieved partial remission (BZ014) or complete remission (BZ015, BZ019, BZ020 and BZ021). e, Percentage of CD45RA+ and CD45RA– subpopulations of in vivo-expanded tEGFR+CD3+ CD19-BBz(86) CAR T cells and tEGFR–CD3+ normal T cells from the six treated patients. P values were calculated from two-tailed Student’s t tests. Horizontal lines denote median values. f, Percentage of CD45RA+CCR7–, CD45RA+CCR7+, CD45RA–CCR7– and CD45RA–CCR7+ subpopulations of in vivo-expanded tEGFR+CD3+ CD19-BBz(86) CAR T cells and tEGFR–CD3+ normal T cells from the six treated patients. P values were calculated from two-tailed Student’s t tests. Horizontal lines denote median values. g–j, Detection of tEGFR+CD3+ CD19-BBz(86) CAR T cells in the peripheral blood of patient BZ015 on day 317 after cell infusion. g, Of the tEGFR+CD3+ CAR T cells, 96.7% were CD8+. h, CD19+ B cells were still depleted. i, 45.8% of the long-term, persistent tEGFR+CD3+ CD19-BBz(86) CAR T cells were CD45RO+CCR7+ central memory T cells in the peripheral blood of patient BZ015 on day 317 after cell infusion, while only 6.25% of the tEGFR–CD3+ normal T cells were CD45RO+CCR7+ central memory T cells. j, qPCR analyses in triplicate also showed long-term persistence of CD19-BBz(86) CAR T cells in peripheral blood.
Supplementary information
Supplementary Information
Supplementary Tables 1–13 and Supplementary Figures 1–16
Supplementary Data 1
CD19-BBz(71) DNA and amino acid sequences
Supplementary Data 2
CD19-BBz(75) DNA and amino acid sequences
Supplementary Data 3
CD19-BBz(82) DNA and amino acid sequences
Supplementary Data 4
CD19-BBz(86) DNA and amino acid sequences
Supplementary Data 5
CD19-BBz(96) DNA and amino acid sequences
Rights and permissions
About this article
Cite this article
Ying, Z., Huang, X.F., Xiang, X. et al. A safe and potent anti-CD19 CAR T cell therapy. Nat Med 25, 947–953 (2019). https://doi.org/10.1038/s41591-019-0421-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-019-0421-7
This article is cited by
-
Current challenges and therapeutic advances of CAR-T cell therapy for solid tumors
Cancer Cell International (2024)
-
Immune modulation in malignant pleural effusion: from microenvironment to therapeutic implications
Cancer Cell International (2024)
-
Programmable synthetic receptors: the next-generation of cell and gene therapies
Signal Transduction and Targeted Therapy (2024)
-
Immunotherapies in rare cancers
Molecular Cancer (2023)
-
Complexities in comparing the impact of costimulatory domains on approved CD19 CAR functionality
Journal of Translational Medicine (2023)