Abstract
Primary tumors may create the premetastatic niche in secondary organs for subsequent metastasis. Humoral immunity contributes to the progression of certain cancers, but the roles of B cells and their derived antibodies in premetastatic niche formation are poorly defined. Using a mouse model of spontaneous lymph node metastasis of breast cancer, we show that primary tumors induced B cell accumulation in draining lymph nodes. These B cells selectively promoted lymph node metastasis by producing pathogenic IgG that targeted glycosylated membrane protein HSPA4, and activated the HSPA4-binding protein ITGB5 and the downstream Src/NF-κB pathway in tumor cells for CXCR4/SDF1α-axis-mediated metastasis. High serum anti-HSPA4 IgG was correlated with high tumor HSPA4 expression and poor prognosis of breast cancer subjects. Our findings identify a key role for tumor-educated B cells and their derived antibodies in lymph node premetastatic niche formation, providing potential targets for cancer intervention.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All requests for raw and analyzed data and materials are promptly reviewed by Second Military Medical University to verify whether the request is subject to any intellectual property or confidentiality obligations. Any data and materials that can be shared will be released via a Material Transfer Agreement. The gene microarray data for B cells and lymph node stromal cells have been deposited in the Gene Expression Omnibus under accession numbers GSE113249 and GSE113250, respectively.
References
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Becker, A. et al. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 30, 836–848 (2016).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Deng, J. et al. S1PR1-STAT3 signaling is crucial for myeloid cell colonization at future metastatic sites. Cancer Cell. 21, 642–654 (2012).
Liu, Y. et al. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell. 30, 243–256 (2016).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Cochran, A. J. et al. Tumour-induced immune modulation of sentinel lymph nodes. Nat. Rev. Immunol. 6, 659–670 (2006).
Iqbal, J., Ginsburg, O., Rochon, P. A., Sun, P. & Narod, S. A. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 313, 165–173 (2015).
Qian, C. N. et al. Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 66, 10365–10376 (2006).
Hirakawa, S. et al. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 109, 1010–1017 (2007).
Harrell, M. I., Iritani, B. M. & Ruddell, A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am. J. Pathol. 170, 774–786 (2007).
Li, Q., Harden, J. L., Anderson, C. D. & Egilmez, N. K. Tolerogenic phenotype of IFN-gamma-induced IDO+ dendritic cells is maintained via an autocrine IDO-kynurenine/AhR-IDO loop. J. Immunol. 197, 962–970 (2016).
Angeli, V. et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 24, 203–215 (2006).
Dougan, S. K. et al. Antigen-specific B-cell receptor sensitizes B cells to infection by influenza virus. Nature 503, 406–409 (2013).
Brodt, P. & Gordon, J. Natural resistance mechanisms may play a role in protection against chemical carcinogenesis. Cancer Immunol. Immunother. 13, 125–127 (1982).
Qin, Z. et al. B cells inhibit induction of T cell-dependent tumor immunity. Nat. Med. 4, 627–630 (1998).
Olkhanud, P. B. et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 71, 3505–3515 (2011).
Yanaba, K. et al. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28, 639–650 (2008).
Schioppa, T. et al. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proc. Natl Acad. Sci. USA 108, 10662–10667 (2011).
Affara, N. I. et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell 25, 809–821 (2014).
Huang, L. et al. CpG-based immunotherapy impairs antitumor activity of BRAF inhibitors in a B-cell-dependent manner. Oncogene 36, 4081–4086 (2017).
Pucci, F. et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science 352, 242–246 (2016).
Lohmueller, J. & Finn, O. J. Current modalities in cancer immunotherapy: Immunomodulatory antibodies, CARs and vaccines. Pharmacol. Ther. 178, 31–47 (2017).
Tan, T. T. & Coussens, L. M. Humoral immunity, inflammation and cancer. Curr. Opin. Immunol. 19, 209–216 (2007).
Andreu, P. et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell 17, 121–134 (2010).
Karagiannis, P. et al. IgG4 subclass antibodies impair antitumor immunity in melanoma. J. Clin. Invest. 123, 1457–1474 (2013).
Carmi, Y. et al. Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature 521, 99–104 (2015).
Shalapour, S. et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 521, 94–98 (2015).
Van den Eynden, G. G. et al. Increased sentinel lymph node lymphangiogenesis is associated with nonsentinel axillary lymph node involvement in breast cancer patients with a positive sentinel node. Clin. Cancer Res. 13, 5391–5397 (2007).
Malhotra, D. et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat. Immunol. 13, 499–510 (2012).
Lee-Chang, C. et al. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J. Immunol. 191, 4141–4151 (2013).
Halama, N. et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell 29, 587–601 (2016).
Zlotnik, A., Burkhardt, A. M. & Homey, B. Homeostatic chemokine receptors and organ-specific metastasis. Nat. Rev. Immunol. 11, 597–606 (2011).
Cui, K. et al. The CXCR4-CXCL12 pathway facilitates the progression of pancreatic cancer via induction of angiogenesis and lymphangiogenesis. J. Surg. Res. 171, 143–150 (2011).
Guan, G. et al. The HIF-1alpha/CXCR4 pathway supports hypoxia-induced metastasis of human osteosarcoma cells. Cancer Lett. 357, 254–264 (2015).
Casadevall, A. & Pirofski, L. A. A new synthesis for antibody-mediated immunity. Nat. Immunol. 13, 21–28 (2011).
Gotoh, K. et al. Apg-2 has a chaperone-like activity similar to Hsp110 and is overexpressed in hepatocellular carcinomas. FEBS Lett. 560, 19–24 (2004).
Kirkegaard, T. et al. Heat shock protein-based therapy as a potential candidate for treating the sphingolipidoses. Sci. Transl. Med. 8, 355ra118 (2016).
Posey, A. D. Jr. et al. Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity 44, 1444–1454 (2016).
Bradbury, A. R., Sidhu, S., Dubel, S. & McCafferty, J. Beyond natural antibodies: the power of in vitro display technologies. Nat. Biotechnol. 29, 245–254 (2011).
Liu, X. et al. Cell surface heat shock protein 90 modulates prostate cancer cell adhesion and invasion through the integrin-beta1/focal adhesion kinase/c-Src signaling pathway. Oncol. Rep. 25, 1343–1351 (2011).
Sidera, K., Gaitanou, M., Stellas, D., Matsas, R. & Patsavoudi, E. A critical role for HSP90 in cancer cell invasion involves interaction with the extracellular domain of HER-2. J. Biol. Chem. 283, 2031–2041 (2008).
Bianchi-Smiraglia, A., Paesante, S. & Bakin, A. V. Integrin beta5 contributes to the tumorigenic potential of breast cancer cells through the Src-FAK and MEK-ERK signaling pathways. Oncogene 32, 3049–3058 (2013).
Kitamura, T., Qian, B. Z. & Pollard, J. W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 15, 73–86 (2015).
Kumar, V., Patel, S., Tcyganov, E. & Gabrilovich, D. I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 37, 208–220 (2016).
Zhou, D. et al. Identification of a pathogenic antibody response to native myelin oligodendrocyte glycoprotein in multiple sclerosis. Proc. Natl Acad. Sci. USA 103, 19057–19062 (2006).
Winer, D. A. et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 17, 610–617 (2011).
de Visser, K. E., Korets, L. V. & Coussens, L. M. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 7, 411–423 (2005).
Liu, H. et al. COX-2-mediated regulation of VEGF-C in association with lymphangiogenesis and lymph node metastasis in lung cancer. Anat. Rec. 293, 1838–1846 (2010).
Garmy-Susini, B. et al. PI3Kalpha activates integrin alpha4beta1 to establish a metastatic niche in lymph nodes. Proc. Natl Acad. Sci. USA 110, 9042–9047 (2013).
Stasinopoulos, I., O’Brien, D. R. & Bhujwalla, Z. M. Inflammation, but not hypoxia, mediated HIF-1alpha activation depends on COX-2. Cancer Biol. Ther. 8, 31–35 (2009).
Jung, Y. J., Isaacs, J. S., Lee, S., Trepel, J. & Neckers, L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 17, 2115–2117 (2003).
Srivastava, P. K., Callahan, M. K. & Mauri, M. M. Treating human cancers with heat shock protein-peptide complexes: the road ahead. Expert. Opin. Biol. Ther. 9, 179–186 (2009).
Garcia-Carbonero, R., Carnero, A. & Paz-Ares, L. Inhibition of HSP90 molecular chaperones: moving into the clinic. Lancet. Oncol. 14, e358–e369 (2013).
Testori, A. et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician’s choice of treatment for stage IV melanoma: the C-100-21 Study Group. J. Clin. Oncol. 26, 955–962 (2008).
Wakisaka, N. et al. Primary tumor-secreted lymphangiogenic factors Induce pre-metastatic lymphvascular niche formation at sentinel lymph nodes in oral squamous cell carcinoma. PLoS One 10, e0144056 (2015).
Pal, S. K. et al. Clinical and translational assessment of VEGFR1 as a mediator of the pre-metastatic niche in high-risk localized prostate cancer. Mol. Cancer Ther. 14, 2896–2900 (2015).
Acharyya, S. et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 150, 165–178 (2012).
Hagglund, P., Bunkenborg, J., Elortza, F., Jensen, O. N. & Roepstorff, P. A new strategy for identification of N-glycosylated proteins and unambiguous assignment of their glycosylation sites using HILIC enrichment and partial deglycosylation. J. Proteome. Res. 3, 556–566 (2004).
Du, F., Bai, Y., Bai, Y. & Liu, H. Quantitative detection of trace systemins in Solanaceous plants by immunoaffinity purification combined with liquid chromatography/electrospray quadrupole time-of-flight mass spectrometry. Anal. Chem. 82, 9374–9383 (2010).
Liu, F. et al. Increased expression of SDF-1/CXCR4 is associated with lymph node metastasis of invasive micropapillary carcinoma of the breast. Histopathology 54, 741–750 (2009).
Acknowledgements
We thank Y. Li and X. Zuo for technical assistance and S. Xu, J. Hou, C. Han, Z. Yu, and Y. Bao for helpful discussions. This work was supported by grants from National Key R&D Program of China (2018YFA0507400) to X.C., the National 135 Major Project of China (2017ZX10203206) to X.C., National Natural Science Foundation of China (81602497 to Y.L., 87188101 to X.C., 91542000 to X.C., 31400757 to Y.G., 91542204 to Y.H., and 81622023 to Y.H.), and CAMS Innovation Fund for Medical Sciences (2016-12M-1-003) to X.C.
Author information
Authors and Affiliations
Contributions
X.C. designed the experiments. Y.G., Y.L., L.F., L.Z., J.Z., Y.H., Y.J., Y.Z., P.Z., Z.J., and X.Z. conducted the experiments. X.C., Y.G., and L.F. analyzed data and wrote the paper. X.C. was responsible for research supervision, coordination, and strategy.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 B cell profiles in peripheral blood, spleen and bone marrow of tumor-bearing mice.
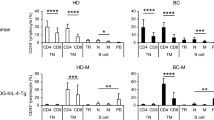
(a) Immunohistochemistry staining of B cells with anti-CD19 antibody in DLN, NDLN and NLN. Scale bars, 500μm. (b) Proportions of CD4 + T cells, CD8 + T cells, dendritic cells (DCs, Ia/e + CD11Chi) and macrophages (CD11bhi F4/80 + ) were detected by flow cytometry in DLNs collected from 0 to 28 days after 4T1 tumor inoculation. (c) The proportions (left) and absolute numbers (right) of B cells in lymph nodes after EMT6 inoculation are shown in linear graph (n = 4 biologically independent animals for each time point). (d) Proportions of B cells (CD19 + ) within mononuclear cells were detected in peripheral blood (d), spleen (h), and bone marrow (l) at day 21 after tumor burden (n = 6 biologically independent animals). (e) Proportions of B cells (CD19 + ) within lymphocytes were detected in peripheral blood (e), spleen (i), and bone marrow (m) at day 21 after tumor burden (n = 6 biologically independent animals). (f) Absolute numbers of B cells (CD19 + ) were detected in peripheral blood (f, per 200 μl volume), spleen (j), and bone marrow (n, per one lower limb) at day 21 after tumor burden. (g) B cell subsets in peripheral blood (g), spleen (k), and bone marrow (o) were detected at day 21 after tumor burden (n = 6 biologically independent animals). Unpaired two-tailed Student’s t-tests for d-o. Data are plotted as mean +/− s.d., which are representative of two (b-o) and three (a) independent replicates.
Extended Data Fig. 2 Characteristics of tumor-induced B cell and LN stromal cells in different lymph nodes.
(a) Semi-qPCR analysis of Igα and luciferase gene expression in lymph node extracts. β-actin was assayed as a control. (b,c) Representative FACS staining of BrdU + B cells (gated on CD19 + ) detected in DLNs, non-DLNs and NLNs at 14 days after BrdU feeding (b), and proportions of BrdU + B cells are also showed in histogram (n = 5 biologically independent animals) (c). Unpaired two-tailed Student’s t-tests. (d) Stromal cells from DLNs or NLNs were pre-coated on the plates, and B cells cultured without serum were seeded on transwell chambers (3 µm pore size). Chemoattractant B cells (CD19 + ) were counted 20 h after incubation by FACS (n = 6 biologically independent samples). Unpaired two-tailed Student’s t-tests. (e) Network of the top 5 increased phosphorylated proteins identified by phosphoprotein profiling with antibody microarray. Red nodes represent phosphorylated proteins with node size for quantity of protein, and green nodes represent related pathways. (f) List of top 20 genes unregulated in DLN B cells vs NLN B cells in the gene chip. The genes with★are immunoglobulin-related. (g) Four subsets of LN stromal cells according to the expression of gp38 and CD31 were detected by flow cytometry. (h) Proportions (h) and quantitation (i) of four subsets of LN stromal cells detected by flow cytometry in NLNs, NDLNs and DLNs (n = 6 biologically independent animals). Unpaired two-tailed Student’s t-tests. Data for c, d, h and i are plotted as mean +/− s.d. Data are representative of three (a-d, g-i) independent replicates.
Extended Data Fig. 3 Surface markers and cytokine production of B cells in different LNs.
(a) Common surface markers of B cells in DLNs, non-DLNs and NLNs analyzed by FACS. (b) IL10, IL6, TNFα, PGE2, TGFβ, IFNγ, IL12p70, and IL2 produced by different B cells stimulated with LPS for 24 h were detected by ELISA (n = 6 biologically independent samples). Data are plotted as mean +/− s.d. Data are representative of three independent replicates.
Extended Data Fig. 4 Immunoglobulin production of different B cells from tumor-bearing mice.
(a,b) λ chain of IgA, IgG1, IgG2a, IgG2b, IgG3, IgM, and IgE produced by different B cells stimulated with lipopolysaccharide (5 μg/ml) for 48 h (n = 6 biologically independent samples) (a) or from mouse serum (n = 8 biologically independent animals) (b) were detected by CBA. (c,d) κ chain (c) and λ chain (d) of IgA, IgG1, IgG2a, IgG2b, IgG3, IgM, and IgE from MMTV-PyMT or WT mouse serum were detected by CBA (n = 6 biologically independent animals). (e) Quantitation of IgG using IgG1, IgG2a, IgG2b, IgG3 ELISA kits for normal and 4T1 tumor-bearing mouse serum (n = 6 biologically independent animals). (f) Quantitation of IgG using ELISA kits for MMTV-PyMT or WT mouse serum (n = 6 biologically independent animals). (g,h) Quantitation of IgG from different LNs (g) or 4T1 tumors (h) (n = 8 biologically independent animals). (i) λ chain of IgG1, IgG2a, IgG2b, IgG3, IgA, IgM, and IgE produced by B cells from peripheral blood (i), spleen (k), and bone marrow (m) with 5 μg/ml lipopolysaccharide stimulation for 48 h were detected by CBA (n = 6 biologically independent animals). (j) κ chain of IgG1, IgG2a, IgG2b, IgG3, IgA, IgM, and IgE produced by B cells from peripheral blood (j), spleen (l), and bone marrow (n) with 5 μg/ml lipopolysaccharide stimulation for 48 h were detected by CBA (n = 6 biologically independent animals). Unpaired two-tailed Student’s t-tests. Data are plotted as mean +/− s.d., which are representative of three independent replicates.
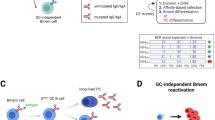
Extended Data Fig. 5 B cells promote breast cancer LN metastasis though pathogenic antibodies.
(a) Qualitative analysis of normal and metastatic LNs from 4T1 tumor-bearing mice by H&E staining. Scale bars, 100 μm. (b) LN metastatic burden of normal or Bnull mice treated with PBS, normal or tumor Ig (n = 12 biologically independent animals). (c) Growth curves of tumors arising from 4T1 tumor cells in each group (n = 12 biologically independent animals). Mammary tumor growth was measured with a digital caliper. (d) Qualitative analysis of metastatic LNs from PyMT or PyMT/Bnull mice treated with PBS, normal or tumor Ig by H&E staining. Scale bars, 100 μm. (e) LN metastatic burden of PyMT or PyMT/Bnull mice treated with PBS, normal or tumor Ig (n = 10 biologically independent animals). (f) Lung metastatic burden of PyMT or PyMT/Bnull mice treated with PBS, normal or tumor immunoglobulin (n = 10 biologically independent animals). (g) Invasion assay of 1 × 105 4T1 cells with or without immunoglobulin stimulation (n = 8 biologically independent samples). (h,i) Representative FACS staining of B cells (CD19 + ) and CD4 + T cells was detected in LNs and spleens after control or anti-CD20 antibody treatment (n = 6 biologically independent animals) (h), and proportions of B cells are also showed in histogram (i). (j) Proportions of regulatory T cell (Tregs), CD8 + T cells, macrophages and dendritic cells (DCs) related to CD4 + T cells are showed in histogram (n = 6 biologically independent animals). Unpaired two-tailed Student’s t-tests. Data are plotted as mean +/− s.d. Data are representative of two (c,h-j) and three (a,b,d-g) independent replicates.
Extended Data Fig. 6 Pathogenic IgG promotes LN metastasis through CXCR4/SDF1α axis.
(a) Related expression of candidate chemokine receptor genes stimulated by IgG was detected by quantitative PCR (n = 6 biologically independent samples). β-actin was assayed as a control. (b) Tumor cell migration assay of 1 × 105 4T1 cells induced by immunoglobulin stimulation with or without RNA silence of CXCR2, CXCR4 and CCR2 (n = 6 biologically independent samples). β-actin was assayed as a control. (c) HIF1α stimulated by IgG for different times (12, 24, 48 h) were detected by immunoblot. β-actin was assayed as a control. (d) Bnull mice were reinfused with either tumor immunoglobulin or ctrl immunoglobulin and inoculated 4T1 tumor cells with or without silence of Hif1α. Representative immunohistochemistry images of HIF1α and related CXCR4 expression stained in tumors from the four groups were shown. Scale bars, 50μm. (e) Expression of PGE2 in tumor cells stimulated by ctrl immunoglobulin or tumor immunoglobulin for different times (12, 24, 48 h) (n = 6 biologically independent samples). (f) Bnull mice were reinfused with either tumor immunoglobulin or ctrl immunoglobulin and inoculated 4T1 tumor cells with or without treatment of COX2 inhibitor celecoxib. Representative immunohistochemistry images of SDF1α expression stained in LNs from the four groups were shown. Scale bars, 50μm. (g) Related expression of Sdf1α in four subsets of stromal cells from DLN, NDLN and NLN was detected by quantitative PCR (n = 6 biologically independent animals). β-actin was assayed as a control. In all panels, data are plotted as mean +/− s.d. and analyzed using unpaired two-tailed Student’s t-tests. Data are representative of three independent replicates.
Extended Data Fig. 7 Pathogenic IgG activates CXCR4/SDF1α axis through NF-κB pathway via binding with HSPA4.
(a) mRNA expression of Cxcr4 in tumor cells pretreated with PDTC, SB203580, SP600125, PD98059, or wortmannin and then stimulated by pathogenic immunoglobulin for 24 h (n = 5 biologically independent samples). (b) CD16/32 expression of 4T1, EMT6 tumor cells and macrophages (positive control) were analyzed by FACS. (c) NF-κB luciferase activation was measured using Dual-Luciferase Reporter Assay system 24 h after transfection (n = 5 biologically independent samples). (d) Binding of whole lysates of 4T1 tumor cells by ctrl or tumor immunoglobulin was detected by western blot. (e) The list of candidate proteins for antigen screening from mass spectrometry analysis. (f) NF-κB luciferase activation was measured using Dual-Luciferase Reporter Assay system 24 h after transfection with or without silence of candidate genes (n = 5 biologically independent samples). (g) mRNA expression of Hspa4, Ncl, Itgb3 and Itgb5 in tumor cells (n = 6 biologically independent samples). (h) HSPA4-Flag and Myc-tagged Ncl, Itgb3, Itgb5 were transfected into tumor cells. Then HSPA4-Flag was immunoprecipitated and other proteins were blotted by anti-Myc antibody. (i) Flag-tagged Hspa4 and Itgb5 were transfected into 4T1 cells. These proteins were immunoprecipitated (IP) with anti-flag antibody and immunoblotted (IB) with normal or tumor immunoglobulin. (j) Immunoblot analysis of HSPA4 level in HSPA4 knock-out (KO) (j) or ITGB5 level in ITGB5−KO (k) tumor cells. (l) Different N-glycosylation sites in the peptide of HSPA4 purified from 4T1, B16/F10 and Hepa 1-6 cells are characterized by mass spectrometry. Red color, underscore, italic represents N-glycosylation sites in 4T1, B16/F10 and Hepa 1-6 cells respectively. (m) λ chain of IgG1, IgG2a, IgG2b, IgG3, IgA, IgM and IgE from the serum of WT or two HSPA4-KO clone bearing mice (n = 8 biologically independent animals) were detected by CBA. (n) Representative immunohistochemistry images of CXCR4, and PGE2 expression in tumors with or without HSPA4 knock out. Scale bars, 50 μm. In all panels, data are plotted as mean +/− s.d. and analyzed using unpaired two-tailed Student’s t-tests. Data are representative of three (a-k, m,n) independent replicates.
Extended Data Fig. 8 ITGB5 knock out impaired CXCR4, PGE2 and SDF1α expression and LN metastasis.
(a) Representative immunohistochemistry images of CXCR4, PGE2 expression in tumors and SDF1α expression in LNs with or without ITGB5 knock out. Scale bars, 50μm. (b) Statistics of metastatic LNs from WT or two ITGB5-KO clone bearing mice (n = 8 biologically independent animals). (c) IgG subclass analysis of anti-HSPA4 immunoglobulin was detected by ELISA (n = 5 biologically independent samples). Data are plotted as mean +/− s.d. and analyzed using unpaired two-tailed Student’s t-tests. Data are representative of three independent replicates.
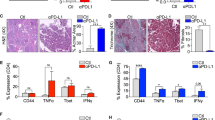
Extended Data Fig. 9 High serum anti-HSPA4 IgG is positively correlated with high tumor cell HSPA4 expression and LN metastasis in breast cancer subjects.
(a) Representative images of negative, weak, median and strong expression of HSPA4 in tumor tissues from subjects with invasive ductal carcinoma (IDC). Scale bars, 50μm. (b) Schematic illustration for the detection of serum anti-HSPA4 IgG levels by enzyme-linked immunosorbent assay (ELISA). (c,d) Representative immunohistochemistry images (c) and comparison (d) of SDF1α expression in benign LNs from breast cancer subjects with (LN + , n = 56 biologically independent subject samples) or without (LN-, n = 52 biologically independent subject samples) LN metastasis. Unpaired two-tailed Student’s t-tests. (e) Immunofluorescent analysis of αSMA and SDF1α expression in LNs from subjects with IDC. Scale bars, 50μm. (f) Representative immunohistochemistry images of HSPA4, CXCR4 expression in tumors and SDF1α expression in LNs from three subjects with IDC. Scale bars, 50μm. Data are representative of three independent replicates for a,c-f.
Extended Data Fig. 10 Working model.
Working model of B cells in the promotion of breast cancer metastasis to lymph node (LN) via pathogenic antibodies which target tumor antigen HSPA4 to activate CXCR4/SDF1α axis in cancer cells through ITGB5 and Src/NF-κB pathway.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2
Source data
Source Data Fig. 3
Unprocessed blots for Figure 3b
Source Data Fig. 4
Unprocessed blots for Figure 4c,e
Source Data Fig. 5
Unprocessed blots for Figure 5a,c,f,g,h,k
Source Data Extended Data Fig. 2
Full-length and unprocessed gels for Extended Data Fig. 2a
Source Data Extended Data Fig. 6
Unprocessed blots for Extended Data Fig. 6c
Source Data Extended Data Fig. 7
Unprocessed blots for Extended Data Fig. 7d,h,I,j,k
Rights and permissions
About this article
Cite this article
Gu, Y., Liu, Y., Fu, L. et al. Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nat Med 25, 312–322 (2019). https://doi.org/10.1038/s41591-018-0309-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-018-0309-y
This article is cited by
-
HSPA4 upregulation induces immune evasion via ALKBH5/CD58 axis in gastric cancer
Journal of Experimental & Clinical Cancer Research (2024)
-
Integrative single-cell transcriptomic analyses reveal the cellular ontological and functional heterogeneities of primary and metastatic liver tumors
Journal of Translational Medicine (2024)
-
Citrullinated fibrinogen-SAAs complex causes vascular metastagenesis
Nature Communications (2023)
-
BACH1-induced ferroptosis drives lymphatic metastasis by repressing the biosynthesis of monounsaturated fatty acids
Cell Death & Disease (2023)
-
Germinal center-dependent and -independent immune responses of tumor-infiltrating B cells in human cancers
Cellular & Molecular Immunology (2023)