Abstract
Photoreceptor ciliopathies constitute the most common molecular mechanism of the childhood blindness Leber congenital amaurosis. Ten patients with Leber congenital amaurosis carrying the c.2991+1655A>G allele in the ciliopathy gene centrosomal protein 290 (CEP290) were treated (ClinicalTrials.gov no. NCT03140969) with intravitreal injections of an antisense oligonucleotide to restore correct splicing. There were no serious adverse events, and vision improved at 3 months. The visual acuity of one exceptional responder improved from light perception to 20/400.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All relevant patient-level data are displayed in the figures. All requests for data will be reviewed by ProQR Therapeutics and the universities involved to verify whether the request is subject to any intellectual property or confidentiality obligations. Patient-related data may be subject to confidentiality. Any data that can be shared will be released.
References
Russell, S. R. et al. Lancet 390, 849–860 (2017).
Jacobson, S. G. et al. Proc. Natl Acad. Sci. USA 102, 6177–6182 (2005).
den Hollander et al. Am. J. Hum. Genet. 79, 556–561 (2006).
Cideciyan, A. V. et al. Hum. Mol. Genet. 20, 1411–1423 (2011).
Jacobson, S. G. et al. Invest. Ophthalmol. Vis. Sci. 58, 2609–2622 (2017).
Dulla, K. et al. Mol. Ther. Nucleic Acids 12, 730–740 (2018).
Jabs, D. A. et al. Am. J. Ophthalmol. 140, 509–516 (2005).
Chew, E. Y. et al. Ophthalmology. 117, 2112–9.e3 (2010).
Cideciyan, A. V. et al. J. Opt. Soc. Am. A 24, 1457–1467 (2007).
Ferris, F. L., Kassoff, A., Bresnick, G. H. & Bailey, I. L. Am. J. Ophthalmol. 94, 91–96 (1982).
Bailey, I. L., Jackson, A. J., Minto, H., Greer, R. B. & Chu, M. A. Optom. Vis. Sci. 89, 1257–1264 (2012).
Roman, A. J., Cideciyan, A. V., Aleman, T. S. & Jacobson, S. G. Physiol. Meas. 28, N51–N56 (2007).
Klein, M. & Birch, D. G. Doc. Ophthalmol. 119, 217–224 (2009).
Collison, F. T., Fishman, G. A., McAnany, J. J., Zernant, J. & Allikmets, R. Retina 34, 1888–1895 (2014).
Shapiro, A. et al. Invest. Ophthalmol. Vis. Sci. 58, abstr. 3290 (2017).
Jacobson, S. G. et al. Hum. Mol. Genet. 22, 168–183 (2013).
Cideciyan, A. V. et al. Invest. Ophthalmol. Vis. Sci. 57, 3211–3221 (2016).
Bates, D., Maechler, M., Bolker, B. & Walker, S. J. Stat. Softw. 67, 1–48 (2015).
Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. J. Stat. Softw. 82, 1–26 (2017).
R v. 3.4.4 https://www.R-project.org/ (The R Project for Statistical Computing, 2018).
Acknowledgements
This work was supported by clinical trial contracts from ProQR Therapeutics to site principal investigators A.V.C., B.P.L., and S.R.R.
Author information
Authors and Affiliations
Contributions
A.V.C. and S.G.J. contributed to the clinical study design and protocol development; performed clinical investigation of patients; reviewed, analyzed, and interpreted the data; and wrote the draft manuscript and its revisions. M.T., M.R.S., P.B., W.d.W., P.A., D.M.R., G.P., and M.D.T. developed the clinical study protocol, reviewed the data, and contributed to all drafts of the manuscript. A.V.D., B.P.L., and S.R.R. performed the clinical investigation of patients, contributed to the clinical study design and protocol development, and contributed to all drafts of the manuscript. A.C.H., F.N., and S.R.R. performed the injections. J.C., A.V.G., A.J.R., A.S., I.C.H., M.D.H., W.L.P., E.H.S., I.B., J.D.Z., and C.V.C. supported clinical investigation of the patients. A.J.R. performed the statistical analyses. P.B., P.A., and M.E.C. performed in vitro experiments determining clinical dosing strategy.
Corresponding authors
Ethics declarations
Competing interests
M.T., M.R.S., P.B., W.d.W., P.A., D.M.R., G.P., and M.D.T. are employees and stock holders of ProQR Therapeutics. M.E.C. was a consultant for ProQR Therapeutics.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 OCT scans along the horizontal meridian crossing the fovea at baseline and months 1 and 3 following the first injection.
Ocular instability prevented imaging in some eyes; in other eyes only a single image per visit was available in the central clinical trial database. na, not available (P9 and P10 have not reached the M3 time point)
Extended Data Fig. 2 En face imaging at baseline and months 1 and 3 following the first injection.
Ocular instability prevented imaging in some or all visits of P1, P5, P6, and P8. Only a single image was available per visit in the central clinical trial database. P9 and P10 have not reached the M3 time point. +, infrared reflectance image obtained instead of infrared autofluorescence image
Extended Data Fig 3 Visual acuity results at baseline and months 1 through 6 following injection(s).
a, Visual acuity results recorded in both eyes of all ten subjects as of the interim analysis cut-off date. Treated eyes are larger up-triangles and untreated contralateral eyes are smaller down-triangles. Minor shifts are introduced to allow visibility of symbols that would otherwise overlap. b, Change in acuity from baseline. The first 3 months of the graph are duplicated in Fig. 1d of the main report. c, Interocular difference (treated eye minus untreated eye) between acuities. Larger symbols in panels b and c are averages from 10 patients at baseline and month 1, 8 patients at months 2 and 3, 6 patients at months 4 and 5, and 4 patients at month 6. Smaller symbols show the individual data points. Error bars, ±1 s.d. Linear mixed-effects models were used for the statistical analysis
Extended Data Fig 4 Oculomotor instability results at baseline, month 3, and month 6 following injection(s).
a,b, Oculomotor instability as log10 of the variation of the radial distance (in mm) of the center of pupil from the mean normal primary gaze locus recorded over a 30-s epoch in a darkened room with (a) or without (b) a bright blue fixation light. Treated eyes are larger up-triangles and untreated contralateral eyes are smaller down-triangles. Minor shifts are introduced to allow visibility of symbols that would otherwise overlap. c,d, Change in instability from baseline. e,f, Interocular difference (treated eye minus untreated eye) between ocular instabilities recorded. Larger symbols in panels b and c are averages from 10 patients at baseline, 7 patients at month 3, and 4 patients at month 6. Smaller symbols show the individual data points. Error bars, ±1 s.d. Linear mixed-effects models were used for the statistical analysis
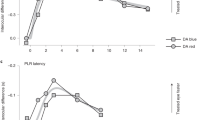
Extended Data Fig 5 FST threshold results at baseline and months 1 through 6 following injection(s).
a,b, FST results recorded in both eyes of all subjects. All data are plotted on the left ordinate scale except for the connected symbols of P7 which are plotted on the integrated luminance scale of the right ordinate. Symbols are averages of repeated FST thresholds obtained within each visit. Nominally, n = 10 repetitions were obtained but there was also some variation (mean = 9.4, mode = 10 samples). c,d, Change in threshold from baseline. The first 3 months of the graphs are duplicated in Fig. 1e,f of the main report. e,f, Interocular difference (treated eye minus untreated eye) between thresholds. Larger symbols in panels c–f are averages from 10 patients at baseline; 9, 8, 10, and 8 patients at month 1; 7, 7, 7, and 7 patients at month 2; 7, 8, 8, and 8 patients at month 3; and 3, 4, 4, and 4 patients at month 6 for untreated red, treated red, untreated blue, and treated blue FSTs, respectively. Smaller symbols show the individual data points. Error bars, ±1 s.d. Linear mixed-effects models were used for the statistical analysis
Extended Data Fig 6 Foveal subcellular anatomy in four of the eight patients who had analyzable recordings at baseline and 3 months in both eyes.
a,b, Longitudinal reflectivity profiles overlaid on foveal OCT scans showing signals associated with intraretinal anatomical features at baseline and month 3 in both eyes. c,d, Changes to outer retinal peaks in terms of backscatter intensity and the peaks used to measure inner (IS) and outer segment (OS) lengths proximal and distal to the connecting cilium, respectively. e,f, Bar graphs of the IS and OS length obtained from a single recording. na, not available due to foveal atrophy
Extended Data Fig 7 Best mobility levels passed at baseline and months 1 through 6 following injection(s).
a, Mobility results recorded in both eyes of all subjects. b, Change in mobility from baseline. c, Interocular difference (treated eye minus untreated eye) between mobility levels. Larger symbols in panels b–c are averages from 9 patients at baseline, 8 patients at month 1, 7 patients at months 2 and 3, and 4 patients at month 6. Error bars, ±1 s.d.
Supplementary information
Supplementary Information
Supplementary Tables 1–5 and Supplementary Note
Rights and permissions
About this article
Cite this article
Cideciyan, A.V., Jacobson, S.G., Drack, A.V. et al. Effect of an intravitreal antisense oligonucleotide on vision in Leber congenital amaurosis due to a photoreceptor cilium defect. Nat Med 25, 225–228 (2019). https://doi.org/10.1038/s41591-018-0295-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-018-0295-0
This article is cited by
-
Full-field stimulus threshold testing: a scoping review of current practice
Eye (2024)
-
Multi-luminance mobility testing after gene therapy in the context of retinal functional diagnostics
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
ISCEV and IPS guideline for the full-field stimulus test (FST)
Documenta Ophthalmologica (2024)
-
Ocular manifestations of renal ciliopathies
Pediatric Nephrology (2024)
-
Detailed analysis of an enriched deep intronic ABCA4 variant in Irish Stargardt disease patients
Scientific Reports (2023)