Abstract
The melanoma genome is dominated by ultraviolet radiation (UVR)-induced mutations. Their relevance in disease progression is unknown. Here we classify melanomas by mutation signatures and identify ten recurrently mutated UVR signature genes that predict patient survival. We validate these findings in primary human melanomas; in mice we show that this signature is imprinted by short-wavelength UVR and that four exposures to UVR are sufficient to accelerate melanomagenesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All sequencing data generated that support the findings of this study have been deposited at the European Genome-phenome Archive (EGA) under accession number EGAS00001003243. All other relevant data are available from the corresponding author upon reasonable request.
Change history
18 December 2018
In the version of this article originally published, Extended Data Fig. 3 was incorrect. A duplicate of Extended Data Fig. 4 was uploaded in place of Extended Data Fig. 3. Extended Data Fig. 3 has now been uploaded. The error has been fixed in the PDF and HTML versions of this article.
References
Whiteman, D. C. et al. J. Natl. Cancer. Inst. 95, 806–812 (2003).
Day, C.-P., Marchalik, R., Merlino, G. & Michael, H. Lab. Invest. 97, 698–705 (2017).
Akbani, R. et al. Cell 161, 1681–1696 (2015).
Hayward, N. K. et al. Nature 545, 175–180 (2017).
Krauthammer, M. et al. Nat. Genet. 44, 1006–1014 (2012).
Brash, D. E. Photochem. Photobiol. 91, 15–26 (2015).
Alexandrov, L. B. et al. Nature 500, 415–421 (2013).
Rosenthal, R., McGranahan, N., Herrero, J., Taylor, B. S. & Swanton, C. Genome Biol. 17, 31 (2016).
Hugo, W. et al. Cell 165, 1–10 (2016).
Riaz, N. et al. Cell 171, 1–16 (2017).
Rooney, M. S., Shukla, S. A., Wu, C. J., Getz, G. & Hacohen, N. Cell 160, 48–61 (2015).
Gentles, A. J. et al. Nat. Med. 21, 938–945 (2015).
El Ghissassi, F. et al. Lancet Oncol. 10, 751–752 (2009).
Dhomen, N. et al. Cancer Cell. 15, 294–303 (2009).
Viros, A. et al. Nature 511, 478–482 (2014).
Whiteman, D. C., Whiteman, C. A. & Green, A. C. Cancer Causes Control 12, 69–82 (2001).
Pollock, P. M. et al. Nat. Genet. 33, 19–20 (2003).
Grossman, R. L. et al. N. Engl. J. Med. 375, 1109–1112 (2016).
Workman, P. et al. Br. J. Cancer 102, 1555–1577 (2010).
Metsalu, T. & Vilo, J. Nucleic Acids Res. 43, W566–W570 (2015).
Jurtz, V. et al. J. Immunol. 199, 3360–3368 (2017).
Charoentong, P. et al. Cell Rep. 18, 248–262 (2017).
Newman, A. M. et al. Nat. Methods 12, 453–457 (2015).
Acknowledgements
This work was supported by CRUK-MI (A27412 and A22902) and the European Research Council (ERC Advanced Grant agreement No. 671262) to R.M. C.G-M. was supported by the French Society of Dermatology, UNICANCER and CEDEF. We acknowledge the contribution of the CRUK-MI core facilities and the contribution of the melanoma specimen donors.
Author information
Authors and Affiliations
Contributions
L.D.T., P.A.M., P.G-M. and R.M. designed the study, analyzed the data and wrote the paper. K.H. designed and performed the experiments and analyzed the data. A.V. and N.D. contributed to study conception and design. A.K.M. and P.A.M. performed the bioinformatics analysis. D.A. provided technical assistance. N.M. and C.G-M. developed the clinical sample cohort. F.B., C.M. and B.S-L. performed the experiments. M.C. and A.V. performed the histological analysis.
Corresponding author
Ethics declarations
Competing interests
R.M. is a consultant for Pfizer. All other authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Signature 7 is associated with improved patient survival.
a,b, Total number of SNVs (a) and proportion of C-to-T transitions at dipyrimidines (b) in non–signature 7 and signature 7 human melanomas from the TCGA dataset. Error bars show mean ± s.d.; n = 419, two-tailed Mann–Whitney test. c, Percentage of C-to-T nucleotide transitions in the TCGA human melanomas presenting wild-type (WT) or mutated (mut) alleles of the indicated genes. Boxes show median (25th–75th percentiles range), whiskers show 5th–95th percentiles range; n = 419, two-tailed Mann–Whitney test. d, Mutation signature for the 10-gene panel displayed as bar graphs showing the fraction of total mutations attributed to each of the 96-trinucleotide mutation types corresponding to a specific substitution and the sequence context adjacent to the mutated base. e, Disease-free survival comparing TCGA patients with non–signature 7 and signature 7 melanomas; n = 365, two-tailed log-rank test. f,g, Overall survival of TCGA patients with melanoma segregated according to signature 7 contribution and stratified by clinical stages I–II (n = 212) (f) and III–IV (n = 165) (g) based on the American Joint Committee on Cancer code (7th edition); two-tailed log-rank test. h, Overall survival of TCGA patients harboring melanomas with a high (top third) or low (bottom third) total number of mutations; n = 273, two-tailed log-rank test. i, Forest plot showing hazard ratio and 95% CI for overall survival for each cut-off value of total number of SNVs defined between the 25th and 75th percentiles; n = 419, *P < 0.05, two-tailed log-rank test. j, Overall survival of cutaneous melanoma patients from combined Hugo, W. et al.9 and Riaz, N. et al.10 cohorts; n = 80, two-tailed log-rank test.
Extended Data Fig. 2 Signature 7 correlates with more predicted neoantigens and a more favourable immune infiltrate composition.
(a) Predicted neoantigen load of melanoma cases from TCGA (ref. 11) segregated into cohorts defined by the contribution of signature 7. (b) Number of predicted SNV-derived neoantigens in non-signature 7 vs. signature 7 human melanomas from TCGA; n = 419, two-tailed Mann-Whitney test. (c) Mutations in the 10-gene panel yielding high-binding affinity putative neoepitopes. (d) Relative leukocyte fractions of 22 immune cell subpopulations inferred from RNA-sequencing data22 using the CIBERSORT deconvolution tool23 in melanomas from TCGA. e,f,g, Distribution of estimated fractions of activated memory CD4 T cell (e), macrophage M1 (f) and monocyte (g) subsets in the non–signature 7 and signature 7 cohorts. Boxes show median (25th–75th percentiles range), whiskers show 5th–95th percentiles range; n = 418, one-tailed Mann–Whitney test.
Extended Data Fig. 3 p53 is induced by short wavelength UVR.
a, Normalized spectral irradiance emitted from the UV280–380, UVB310–315 and UVA350–400 lamps. b, Schematic representation of experimental design. c, Photomicrographs of IHC for p53 in the protected and contralateral UV280–380, UVB310–315 and UVA350–400 exposed skins of BRAFV600E-expressing mice 24 h after exposure. Scale bar, 30 μm. Representative of n = 5 animals per group.
Extended Data Fig. 4 Melanomagenesis is induced by short wavelength UVR.
a, Schematic showing the experimental approach. In the chronic UVR cohorts, BRAFV600E was expressed in the melanocytes of 8-week-old mice using Cre-recombinase/LoxP technology. Four weeks later, mice were treated with UVR (UV280–380, UVB310–315 or UVA350–400) weekly for up to 26 weeks (chronic exposure). b, Photomicrographs of H&E-stained skin sections from protected and UVR-exposed sides of the back of animals treated with UVB310–315 or UVA350–400. Scale bar, 300 µm. Right side panels are magnifications of boxed areas. Scale bar, 25 μm. Representative of all animals (n = 40) included in both cohorts. c, Photographs showing the macroscopic appearance of the skin of protected and UVR-exposed BRAFV600E mice when they reached tumour volume limits. Dashed lines divide the UVR protected (P) and exposed (E) areas. Individual tumors are highlighted by dotted lines. Representative of all animals (n = 87) included in each cohort. d,e, Schematics showing the experimental approach. For the short-term UV280–380 cohort (d), BRAFV600E expression was induced at 8–12 weeks of age, and 4 weeks later mice were treated with UVR weekly for 4 weeks. For the UV280–380 BRAF nave cohort (e), 8-week-old mice were exposed to UVR weekly for 4 weeks, followed by induction of BRAFV600E a week later.
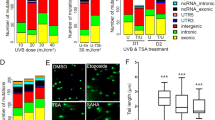
Extended Data Fig. 5 Signature 7 is imprinted by short wavelength UVR.
a, Examples of mutation signatures in mouse melanomas. Bar graphs show the fraction of total mutations attributed to each of the 96-trinucleotide mutation types corresponding to a specific substitution and the sequence context adjacent to the mutated base. Representative of all (n = 78) sequenced tumours. b,c, Total number of SNVs (b) and proportion of C-to-T transitions at dipyrimidines (c) in non–signature 7 and signature 7 BRAFV600E murine melanomas coloured by experimental cohort; n = 78, two-tailed Mann–Whitney test. d, Predicted neoantigen load of mouse melanomas. e, Number of predicted SNV-derived neoantigens in non–signature 7 versus signature 7 mouse melanomas; n = 78, two-tailed Mann–Whitney test. f, Mutations in the 10-gene panel yielding high-binding affinity putative neoepitopes.
Supplementary information
Supplementary Tables
Supplementary Tables 1–11
Rights and permissions
About this article
Cite this article
Trucco, L.D., Mundra, P.A., Hogan, K. et al. Ultraviolet radiation–induced DNA damage is prognostic for outcome in melanoma. Nat Med 25, 221–224 (2019). https://doi.org/10.1038/s41591-018-0265-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-018-0265-6
This article is cited by
-
Refining mutanome-based individualised immunotherapy of melanoma using artificial intelligence
European Journal of Medical Research (2024)
-
Mutational signatures reveal mutual exclusivity of homologous recombination and mismatch repair deficiencies in colorectal and stomach tumors
Scientific Data (2023)
-
DNA damage and somatic mutations in mammalian cells after irradiation with a nail polish dryer
Nature Communications (2023)
-
The journey from melanocytes to melanoma
Nature Reviews Cancer (2023)
-
Pan-cancer T cell atlas links a cellular stress response state to immunotherapy resistance
Nature Medicine (2023)