Abstract
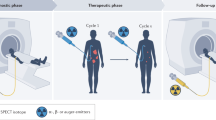
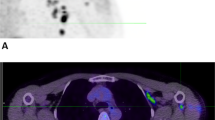
Programmed cell death protein-1/ligand-1 (PD-1/PD-L1) blockade is effective in a subset of patients with several tumor types, but predicting patient benefit using approved diagnostics is inexact, as some patients with PD-L1-negative tumors also show clinical benefit1,2. Moreover, all biopsy-based tests are subject to the errors and limitations of invasive tissue collection3,4,5,6,7,8,9,10,11. Preclinical studies of positron-emission tomography (PET) imaging with antibodies to PD-L1 suggested that this imaging method might be an approach to selecting patients12,13. Such a technique, however, requires substantial clinical development and validation. Here we present the initial results from a first-in-human study to assess the feasibility of imaging with zirconium-89-labeled atezolizumab (anti-PD-L1), including biodistribution, and secondly test its potential to predict response to PD-L1 blockade (ClinicalTrials.gov identifiers NCT02453984 and NCT02478099). We imaged 22 patients across three tumor types before the start of atezolizumab therapy. The PET signal, a function of tracer exposure and target expression, was high in lymphoid tissues and at sites of inflammation. In tumors, uptake was generally high but heterogeneous, varying within and among lesions, patients, and tumor types. Intriguingly, clinical responses in our patients were better correlated with pretreatment PET signal than with immunohistochemistry- or RNA-sequencing-based predictive biomarkers, encouraging further development of molecular PET imaging for assessment of PD-L1 status and clinical response prediction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The RNA-sequencing dataset presented in this manuscript is available through GEO (series accession GSE115594). The data are annotated with a short summary and a description of the study design and can be freely downloaded via the GEO website (https://www.ncbi.nlm.nih.gov/geo/). Clinical details of the cases and laboratory data, restricted to non-identifying data owing to privacy concerns, can be requested by e-mail from the corresponding author, who will handle all requests.
References
Balar, A. V. et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389, 67–76 (2017).
Daud, A. I. et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J. Clin. Oncol. 34, 4102–4109 (2016).
Hegde, P. S., Karanikas, V. & Evers, S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin. Cancer Res. 22, 1865–1874 (2016).
Rosenberg, J. E. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387, 1909–1920 (2016).
Chen, P. L. et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 6, 827–837 (2016).
Ansell, S. M. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 372, 311–319 (2015).
Dong, Z. Y. et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin. Cancer Res. 23, 3012–3024 (2016).
Fehrenbacher, L. et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387, 1837–1846 (2016).
Kowanetz, M. et al. Tumor mutation load assessed by FoundationOne (FM1) is associated with improved efficacy of atezolizumab (atezo) in patients with advanced NSCLC. Ann. Oncol. 27, 15–42 (2016).
Van Allen, E. M. et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350, 207–211 (2015).
Kim, J. M. & Chen, D. S. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann. Oncol. 27, 1492–1504 (2016).
Heskamp, S. et al. Noninvasive imaging of tumor PD-L1 expression using radiolabeled anti-PD-L1 antibodies. Cancer Res. 75, 2928–2936 (2015).
Chatterjee, S. et al. A humanized antibody for imaging immune checkpoint ligand PD-L1 expression in tumors. Oncotarget 7, 10215–10227 (2016).
Rehman, J. A. et al. Quantitative and pathologist-read comparison of the heterogeneity of programmed death-ligand 1 (PD-L1) expression in non-small cell lung cancer. Modern Pathol. 30, 340–349 (2017).
Smith, J. et al. Quantitative and qualitative characterization of two PD-L1 clones: SP263 and E1L3N. Diagn. Pathol. 11, 44 (2016).
McLaughlin, J. et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol. 2, 46–54 (2016).
Scheel, A. H. et al. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Modern Pathol. 29, 1165–1172 (2016).
Madore, J. et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigm. Cell Melanoma Res. 28, 245–253 (2015).
Sanmamed, M. F. & Chen, L. Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J. 20, 256–261 (2014).
Gniadek, T. J. et al. Heterogeneous expression of PD-L1 in pulmonary squamous cell carcinoma and adenocarcinoma: implications for assessment by small biopsy. Modern Pathol. 30, 530–538 (2017).
McDermott, D. F. et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J. Clin. Oncol. 34, 833–842 (2016).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Dijkers, E. C. et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 87, 586–592 (2010).
Gaykema, S. B. et al. 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin. Cancer Res. 20, 3945–3954 (2014).
Lamberts, T. E. et al. ImmunoPET with anti-mesothelin antibody in patients with pancreatic and ovarian cancer before anti-mesothelin antibody-drug conjugate treatment. Clin. Cancer Res. 22, 1642–1652 (2016).
den Hollander, M. W. et al. TGF-β antibody uptake in recurrent high-grade glioma imaged with 89Zr-fresolimumab PET. J. Nucl. Med. 56, 1310–1314 (2015).
Ogembo, J. G. et al. SIRPα and FHOD1 are unique markers of littoral cells, a recently evolved major cell population of red pulp of human spleen. J. Immunol. 188, 4496–4505 (2012).
Fullerton, J. N. & Gilroy, D. W. Resolution of inflammation: a new therapeutic frontier. Nat. Rev. Drug Discov. 15, 551–567 (2016).
Johnson, D. B., Rioth, M. J. & Horn, L. Immune checkpoint inhibitors in NSCLC. Curr. Treat. Options Oncol. 15, 658–669 (2014).
Powles, T. et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515, 558–562 (2014).
Petrylak, D. P. et al. Atezolizumab (MPDL3280A) monotherapy for patients with metastatic urothelial cancer: long-term outcomes from a phase 1 study. JAMA Oncol. 4, 537–544 (2018).
Emens, L. et al. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer (TNBC). Cancer Res. 75, abstr. 2859 (2015).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Lamberts, L. E. et al. Antibody positron emission tomography imaging in anticancer drug development. J. Clin. Oncol. 33, 1491–1504 (2015).
Verel, I. et al. 89Zr immuno-PET: comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J. Nucl. Med. 44, 1271–1281 (2003).
Terwisscha van Scheltinga, A. G. et al. ImmunoPET and biodistribution with human epidermal growth factor receptor 3 targeting antibody 89Zr-RG7116. mABs 6, 1051–1058 (2014).
Oude Munnink, T. H. et al. PET with the 89Zr-labeled transforming growth factor-beta antibody fresolimumab in tumor models. J. Nucl. Med. 52, 2001–2008 (2011).
Makris, N. E. et al. Multicenter harmonization of 89Zr PET/CT performance. J. Nucl. Med. 55, 264–267 (2014).
Frings, V. et al. Repeatability of metabolically active tumor volume measurements with FDG PET/CT in advanced gastrointestinal malignancies: a multicenter study. Radiology 273, 539–548 (2014).
U.S. Department of Health And Human Services. Common terminology criteria for adverse events (CTCAE) version 4.0 (NIH publication no. 09-7473) (National Cancer Institute, Bethesda, MD, USA, 2009).
Austin, P. C. & Stuart, E. A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 34, 3661–3679 (2015).
Acknowledgements
We thank patients and their families for participating in this study. This work was supported by the Dutch Cancer Society grant RUG 2016-10034 (POINTING) and the ERC Advanced grant OnQview ERC 293445, both awarded to E.G.E.d.V., a personal Dutch Cancer Society fellowship RUG 2014-6625 awarded to F.B., and a research grant from Hoffmann–La Roche/Genentech, which was made available to the UMCG.
Author information
Authors and Affiliations
Contributions
F.B., E.G.E.d.V., B.M.F., and A.d.C. designed the study. F.B., E.L.v.d.V., M.N.L.-d.H., A.J.-S., R.B., S.G.E., B.M.F., C.M., and A.d.C. developed the methodology. Acquisition of data was performed by F.B., C.M., T.C.K., E.L.v.d.V., I.C.K., S.F.O., C.P.S., T.J.N.H., A.J.v.d.W., H.J.M.G., J.A.G., A.H.B., and S.S.B. S.G.E., F.B., C.M., E.L.v.d.V., and S.-P.W. conducted statistical analyses and preclinical experiments. A.H.B. and E.G.E.d.V. provided supervision. F.B., E.G.E.d.V., B.M.F., S.-P.W., S.S.B., C.M., and S.G.E. wrote the manuscript. Results were discussed by all authors, who also commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following as competing interests: H.J.M.G. received research support from Hoffmann–La Roche (payment to the institution) and has an advisory role for Roche Netherlands; B.M.F., C.M., S.S.B., A.d.C., and S.-P.W. are employed by Hoffman–La Roche/Genentech and own stock in Hoffman–La Roche/Genentech; E.G.E.d.V. received research support from Hoffman–La Roche/Genentech (payment to the institution) and is a member of the ESMO Magnitude of Clinical Benefit Scale.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Tables 1–6
Rights and permissions
About this article
Cite this article
Bensch, F., van der Veen, E.L., Lub-de Hooge, M.N. et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med 24, 1852–1858 (2018). https://doi.org/10.1038/s41591-018-0255-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-018-0255-8
This article is cited by
-
ImmunoPET imaging of Trop2 in patients with solid tumours
EMBO Molecular Medicine (2024)
-
Development of small-molecular-based radiotracers for PET imaging of PD-L1 expression and guiding the PD-L1 therapeutics
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
The role of radiotherapy in tumor immunity and the potential of PET/CT in detecting the expression of PD-1/PD-L1
Japanese Journal of Radiology (2024)
-
[68Ga]Ga-AUNP-12 PET imaging to assess the PD-L1 status in preclinical and first-in-human study
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Potential non-invasive biomarkers in tumor immune checkpoint inhibitor therapy: response and prognosis prediction
Biomarker Research (2023)