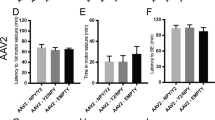
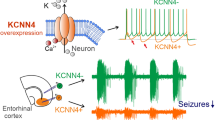
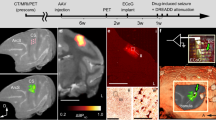
Abstract
Despite the introduction of more than one dozen new antiepileptic drugs in the past 20 years, approximately one-third of people who develop epilepsy continue to have seizures on mono- or polytherapy1. Viral-vector-mediated gene transfer offers the opportunity to design a rational treatment that builds on mechanistic understanding of seizure generation and that can be targeted to specific neuronal populations in epileptogenic foci2. Several such strategies have shown encouraging results in different animal models, although clinical translation is limited by possible effects on circuits underlying cognitive, mnemonic, sensory or motor function. Here, we describe an autoregulatory antiepileptic gene therapy, which relies on neuronal inhibition in response to elevations in extracellular glutamate. It is effective in a rodent model of focal epilepsy and is well tolerated, thus lowering the barrier to clinical translation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chen, Z., Brodie, M. J., Liew, D. & Kwan, P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 75, 279–286 (2018).
Kullmann, D. M., Schorge, S., Walker, M. C. & Wykes, R. C. Gene therapy in epilepsy-is it time for clinical trials? Nat. Rev. Neurol. 10, 300–304 (2014).
Kwan, P., Schachter, S. C. & Brodie, M. J. Drug-resistant epilepsy. N. Engl. J. Med. 365, 919–926 (2011).
Tang, F., Hartz, A. M. S. & Bauer, B. Drug-resistant epilepsy: multiple hypotheses, few answers. Front. Neurol. 8, 301 (2017).
Perucca, P. & Gilliam, F. G. Adverse effects of antiepileptic drugs. Lancet Neurol. 11, 792–802 (2012).
Ryvlin, P., Cross, J. H. & Rheims, S. Epilepsy surgery in children and adults. Lancet Neurol. 13, 1114–1126 (2014).
Wykes, R. C. et al. Optogenetic and potassium channel gene therapy in a rodent model of focal neocortical epilepsy. Sci. Transl. Med. 4, 161ra152 (2012).
Kätzel, D., Nicholson, E., Schorge, S., Walker, M. C. & Kullmann, D. M. Chemical-genetic attenuation of focal neocortical seizures. Nat. Commun. 5, 3847 (2014).
Cook, M. J. et al. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study. Lancet Neurol. 12, 563–571 (2013).
Baldassano, S. N. et al. Crowdsourcing seizure detection: algorithm development and validation on human implanted device recordings. Brain 140, 1680–1691 (2017).
Buckingham, S. C. et al. Glutamate release by primary brain tumors induces epileptic activity. Nat. Med. 17, 1269–1274 (2011).
During, M. J. & Spencer, D. D. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet 341, 1607–1610 (1993).
Stephens, M. L. et al. Tonic glutamate in CA1 of aging rats correlates with phasic glutamate dysregulation during seizure. Epilepsia 55, 1817–1825 (2014).
Cavus, I. et al. 50 Hz hippocampal stimulation in refractory epilepsy: higher level of basal glutamate predicts greater release of glutamate. Epilepsia 57, 288–297 (2016).
Thomas, P. M., Phillips, J. P. & O’Connor, W. T. Hippocampal microdialysis during spontaneous intraoperative epileptiform activity. Acta Neurochir. (Wien.) 146, 143–151 (2004).
Frazier, S. J., Cohen, B. N. & Lester, H. A. An engineered glutamate-gated chloride (GluCl) channel for sensitive, consistent neuronal silencing by ivermectin. J. Biol. Chem. 288, 21029–21042 (2013).
Yaguchi, M. et al. Characterization of the properties of seven promoters in the motor cortex of rats and monkeys after lentiviral vector-mediated gene transfer. Hum. Gene Ther. Methods 24, 333–344 (2013).
Barker-Haliski, M. & White, H. S. Glutamatergic mechanisms associated with seizures and epilepsy. Cold Spring Harb. Perspect. Med. 5, a022863 (2015).
Tzingounis, A. V. & Wadiche, J. I. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat. Rev. Neurosci. 8, 935–947 (2007).
Vink, C. A. et al. Eliminating HIV-1 packaging sequences from lentiviral vector proviruses enhances safety and expedites gene transfer for gene therapy. Mol. Ther. 25, 1790–1804 (2017).
Mainardi, M., Pietrasanta, M., Vannini, E., Rossetto, O. & Caleo, M. Tetanus neurotoxin-induced epilepsy in mouse visual cortex. Epilepsia 53, e132–e136 (2012).
Cleeren, E., Casteels, C., Goffin, K., Janssen, P. & Van Paesschen, W. Ictal perfusion changes associated with seizure progression in the amygdala kindling model in the rhesus monkey. Epilepsia 56, 1366–1375 (2015).
Weir, G. A. et al. Using an engineered glutamate-gated chloride channel to silence sensory neurons and treat neuropathic pain at the source. Brain 140, 2570–2585 (2017).
Jaenisch, N. et al. Reduced tonic inhibition after stroke promotes motor performance and epileptic seizures. Sci. Rep. 6, 26173 (2016).
Asztely, F., Erdemli, G. & Kullmann, D. M. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron 18, 281–293 (1997).
Cavus, I. et al. Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann. Neurol. 57, 226–235 (2005).
Miles, R., Blaesse, P., Huberfeld, G., Wittner, L. & Kaila, K. Chloride homeostasis and GABA signaling in temporal lobe epilepsy. in Jasper’s Basic Mechanisms of the Epilepsies (eds. Noebels, J. L. et al.) (National Center for Biotechnology Information, Bethesda, MD, USA, 2012).
Bolte, S. & Cordelières, F. P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 (2006).
Acknowledgements
We thank G. Schiavo (UCL Institute of Neurology) for the gift of tetanus toxin and S. Hart (UCL Institute of Child Health) for the mouse Neuro-2a cell line. We are grateful to J. Cornford for assistance with ECoG analysis and to K. Hashemi for help optimizing wireless-transmitter use. This project was supported by the European Union’s Horizon 2020 research and innovation program (Marie Skłodowska-Curie grant agreement no. 701411 to A.L.); the Medical Research Council (MR/L01095X/1 to D.M.K., S.S. and M.C.W.); and the Wellcome Trust (095580/Z/11/Z to D.M.K.; 104033/Z/14/Z to D.M.K. and S.S.).
Author information
Authors and Affiliations
Contributions
A.L. and D.M.K. designed all experiments and drafted the manuscript. A.L. performed in vitro electrophysiology and in vivo experiments. A.L. and Y.Q. designed, performed and analyzed in vivo behavioral experiments. Y.Q., A.L., J.P.H. and C.L.D. performed and analyzed all immunostaining experiments. A.L., Y.Q., J.P.H., C.L.D., M.C.W., S.S. and D.M.K. revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4
Supplementary Video 1
Representative chemoconvulsant-induced seizure
Supplementary Video 2
Representative seizure in the model of chronic focal neocortical epilepsy
Rights and permissions
About this article
Cite this article
Lieb, A., Qiu, Y., Dixon, C.L. et al. Biochemical autoregulatory gene therapy for focal epilepsy. Nat Med 24, 1324–1329 (2018). https://doi.org/10.1038/s41591-018-0103-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-018-0103-x
This article is cited by
-
Acetylcholine receptor based chemogenetics engineered for neuronal inhibition and seizure control assessed in mice
Nature Communications (2024)
-
‘On-demand’ gene therapy for epilepsy
Nature Reviews Neurology (2023)
-
Advancement in CRISPR/Cas9 Technology to Better Understand and Treat Neurological Disorders
Cellular and Molecular Neurobiology (2023)
-
Gene Therapy Approach with an Emphasis on Growth Factors: Theoretical and Clinical Outcomes in Neurodegenerative Diseases
Molecular Neurobiology (2022)
-
Bringing balance to the force–regulatable gene therapy for epilepsy
Gene Therapy (2019)