Abstract
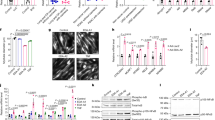
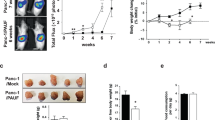
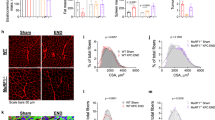
Patients with metastatic cancer experience a severe loss of skeletal muscle mass and function known as cachexia. Cachexia is associated with poor prognosis and accelerated death in patients with cancer, yet its underlying mechanisms remain poorly understood. Here, we identify the metal-ion transporter ZRT- and IRT-like protein 14 (ZIP14) as a critical mediator of cancer-induced cachexia. ZIP14 is upregulated in cachectic muscles of mice and in patients with metastatic cancer and can be induced by TNF-α and TGF-β cytokines. Strikingly, germline ablation or muscle-specific depletion of Zip14 markedly reduces muscle atrophy in metastatic cancer models. We find that ZIP14-mediated zinc uptake in muscle progenitor cells represses the expression of MyoD and Mef2c and blocks muscle-cell differentiation. Importantly, ZIP14-mediated zinc accumulation in differentiated muscle cells induces myosin heavy chain loss. These results highlight a previously unrecognized role for altered zinc homeostasis in metastatic cancer–induced muscle wasting and implicate ZIP14 as a therapeutic target for its treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lambert, A. W., Pattabiraman, D. R. & Weinberg, R. A. Emerging biological principles of metastasis. Cell 168, 670–691 (2017).
Massagué, J. & Obenauf, A. C. Metastatic colonization by circulating tumour cells. Nature 529, 298–306 (2016).
Waning, D. L. et al. Excess TGF-β mediates muscle weakness associated with bone metastases in mice. Nat. Med. 21, 1262–1271 (2015).
Becker, A. et al. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 30, 836–848 (2016).
McAllister, S. S. & Weinberg, R. A. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat. Cell Biol. 16, 717–727 (2014).
Argilés, J. M., Busquets, S., Stemmler, B. & López-Soriano, F. J. Cancer cachexia: understanding the molecular basis. Nat. Rev. Cancer 14, 754–762 (2014).
Fearon, K. C., Glass, D. J. & Guttridge, D. C. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 16, 153–166 (2012).
Fearon, K., Arends, J. & Baracos, V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 10, 90–99 (2013).
Baracos, V. E., Martin, L., Korc, M., Guttridge, D. C. & Fearon, K. C. H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 4, 17105 (2018).
Cohen, S., Nathan, J. A. & Goldberg, A. L. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 14, 58–74 (2015).
Sandri, M. Protein breakdown in cancer cachexia. Semin. Cell Dev. Biol. 54, 11–19 (2016).
Penna, F., Busquets, S. & Argilés, J. M. Experimental cancer cachexia: evolving strategies for getting closer to the human scenario. Semin. Cell Dev. Biol. 54, 20–27 (2016).
Dewys, W. D. et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Am. J. Med. 69, 491–497 (1980).
Aydemir, T. B. & Cousins, R. J. The multiple faces of the metal transporter ZIP14 (SLC39A14). J. Nutr. 148, 174–184 (2018).
Finney, L. A. & O’Halloran, T. V. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 300, 931–936 (2003).
Lichten, L. A. & Cousins, R. J. Mammalian zinc transporters: nutritional and physiologic regulation. Annu. Rev. Nutr. 29, 153–176 (2009).
Larsson, S., Karlberg, I., Selin, E., Daneryd, P. & Peterson, H. I. Trace element changes in serum and skeletal muscle compared to tumour tissue in sarcoma-bearing rats. In Vivo 1, 131–140 (1987).
Siren, P. M. & Siren, M. J. Systemic zinc redistribution and dyshomeostasis in cancer cachexia. J. Cachexia Sarcopenia Muscle 1, 23–33 (2010).
Talmadge, J. E. & Fidler, I. J. Enhanced metastatic potential of tumor cells harvested from spontaneous metastases of heterogeneous murine tumors. J. Natl. Cancer Inst. 69, 975–980 (1982).
Francia, G., Cruz-Munoz, W., Man, S., Xu, P. & Kerbel, R. S. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat. Rev. Cancer 11, 135–141 (2011).
Fearon, K. C. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur. J. Cancer 44, 1124–1132 (2008).
Azoulay, E. et al. The prognosis of acute respiratory failure in critically ill cancer patients. Medicine (Baltimore) 83, 360–370 (2004).
Iguchi, H., Onuma, E., Sato, K., Sato, K. & Ogata, E. Involvement of parathyroid hormone–related protein in experimental cachexia induced by a human lung cancer–derived cell line established from a bone metastasis specimen. Int. J. Cancer 94, 24–27 (2001).
Shum, A. M. et al. Cardiac and skeletal muscles show molecularly distinct responses to cancer cachexia. Physiol. Genomics 47, 588–599 (2015).
Bonetto, A. et al. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS One 6, e22538 (2011).
Kwon, M. C. & Berns, A. Mouse models for lung cancer. Mol. Oncol. 7, 165–177 (2013).
Nguyen, D. X. et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell 138, 51–62 (2009).
Xu, C. et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell 25, 590–604 (2014).
Balkwill, F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev. 25, 409–416 (2006).
Esposito, M., Guise, T. & Kang, Y. The biology of bone metastasis. Cold Spring Harb. Perspect. Med. a031252 (2017).
Cai, D. et al. IKKβ/NF-κβ activation causes severe muscle wasting in mice. Cell 119, 285–298 (2004).
Guttridge, D. C., Mayo, M. W., Madrid, L. V., Wang, C. Y. & Baldwin, A. S. Jr. NF-κβ-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 289, 2363–2366 (2000).
Hojyo, S. et al. The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor–mediated signaling required for systemic growth. PLoS One 6, e18059 (2011).
Liuzzi, J. P. et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA 102, 6843–6848 (2005).
He, W. A. et al. NF-κB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J. Clin. Invest. 123, 4821–4835 (2013).
Sabourin, L. A. & Rudnicki, M. A. The molecular regulation of myogenesis. Clin. Genet. 57, 16–25 (2000).
Penna, F. et al. Muscle wasting and impaired myogenesis in tumor bearing mice are prevented by ERK inhibition. PLoS One 5, e13604 (2010).
Liu, N. et al. Requirement of MEF2A, C, and D for skeletal muscle regeneration. Proc. Natl. Acad. Sci. USA 111, 4109–4114 (2014).
Skapek, S. X., Rhee, J., Spicer, D. B. & Lassar, A. B. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science 267, 1022–1024 (1995).
Wei, Q. & Paterson, B. M. Regulation of MyoD function in the dividing myoblast. FEBS Lett. 490, 171–178 (2001).
Glass, D. J. Signaling pathways perturbing muscle mass. Curr. Opin. Clin. Nutr. Metab. Care 13, 225–229 (2010).
Roberts, B. M. et al. Diaphragm and ventilatory dysfunction during cancer cachexia. FASEB J. 27, 2600–2610 (2013).
Cohen, S. et al. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J. Cell Biol. 185, 1083–1095 (2009).
Clarke, B. A. et al. The E3 ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 6, 376–385 (2007).
Acharyya, S. et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J. Clin. Invest. 114, 370–378 (2004).
Gupta, S. K., Shukla, V. K., Vaidya, M. P., Roy, S. K. & Gupta, S. Serum and tissue trace elements in colorectal cancer. J. Surg. Oncol. 52, 172–175 (1993).
Russell, S. T., Siren, P. M., Siren, M. J. & Tisdale, M. J. The role of zinc in the anti-tumour and anti-cachectic activity of d-myo-inositol 1,2,6-triphosphate. Br. J. Cancer 102, 833–836 (2010).
Cousins, R. J. & Leinart, A. S. Tissue-specific regulation of zinc metabolism and metallothionein genes by interleukin 1. FASEB J. 2, 2884–2890 (1988).
Summermatter, S. et al. Blockade of metallothioneins 1 and 2 increases skeletal muscle mass and strength. Mol. Cell. Biol. 37, e00305–16 (2017).
Crawford, A. J. & Bhattacharya, S. K. Excessive intracellular zinc accumulation in cardiac and skeletal muscles of dystrophic hamsters. Exp. Neurol. 95, 265–276 (1987).
Lecker, S. H. et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 18, 39–51 (2004).
Kang, Y. et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc. Natl. Acad. Sci. USA 102, 13909–13914 (2005).
Schiaffino, S., Dyar, K. A., Ciciliot, S., Blaauw, B. & Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 280, 4294–4314 (2013).
Eley, H. L., Skipworth, R. J., Deans, D. A., Fearon, K. C. & Tisdale, M. J. Increased expression of phosphorylated forms of RNA-dependent protein kinase and eukaryotic initiation factor 2α may signal skeletal muscle atrophy in weight-losing cancer patients. Br. J. Cancer 98, 443–449 (2008).
Schmitt, T. L. et al. Activity of the Akt-dependent anabolic and catabolic pathways in muscle and liver samples in cancer-related cachexia. J. Mol. Med. (Berl.) 85, 647–654 (2007).
Yamasaki, S. et al. Zinc is a novel intracellular second messenger. J. Cell Biol. 177, 637–645 (2007).
Andreini, C., Bertini, I. & Rosato, A. Metalloproteomes: a bioinformatic approach. Acc. Chem. Res. 42, 1471–1479 (2009).
Dumont, N. A., Wang, Y. X. & Rudnicki, M. A. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 142, 1572–1581 (2015).
Talbert, E. E. & Guttridge, D. C. Impaired regeneration: a role for the muscle microenvironment in cancer cachexia. Semin. Cell Dev. Biol. 54, 82–91 (2016).
Wallace, G. Q. & McNally, E. M. Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu. Rev. Physiol. 71, 37–57 (2009).
Jahchan, N. S. et al. A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer Discov. 3, 1364–1377 (2013).
Acharyya, S. et al. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell 8, 421–432 (2005).
Blanco, M. A. et al. Global secretome analysis identifies novel mediators of bone metastasis. Cell Res. 22, 1339–1355 (2012).
Polge, C. et al. Muscle actin is polyubiquitinylated in vitro and in vivo and targeted for breakdown by the E3 ligase MuRF1. FASEB J. 25, 3790–3802 (2011).
Gee, K. R., Zhou, Z. L., Qian, W. J. & Kennedy, R. Detection and imaging of zinc secretion from pancreatic β-cells using a new fluorescent zinc indicator. J. Am. Chem. Soc. 124, 776–778 (2002).
Dalal, B. I., Keown, P. A. & Greenberg, A. H. Immunocytochemical localization of secreted transforming growth factor-β1 to the advancing edges of primary tumors and to lymph node metastases of human mammary carcinoma. Am. J. Pathol. 143, 381–389 (1993).
Jenkitkasemwong, S. et al. slc39a14 is required for the development of hepatocellular iron overload in murine models of hereditary hemochromatosis. Cell Metab. 22, 138–150 (2015).
Burkholder, T., Foltz, C., Karlsson, E., Linton, C. G. & Smith, J. M. Health evaluation of experimental laboratory mice. Curr. Protoc. Mouse Biol. 2, 145–165 (2012).
DuPage, M., Dooley, A. L. & Jacks, T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat. Protoc. 4, 1064–1072 (2009).
Ohly, P., Dohle, C., Abel, J., Seissler, J. & Gleichmann, H. Zinc sulphate induces metallothionein in pancreatic islets of mice and protects against diabetes induced by multiple low doses of streptozotocin. Diabetologia 43, 1020–1030 (2000).
Buclez, P. O. et al. Rapid, scalable, and low-cost purification of recombinant adeno-associated virus produced by baculovirus expression vector system. Mol. Ther. Methods Clin. Dev. 3, 16035 (2016).
Vogler, T. O., Gadek, K. E., Cadwallader, A. B., Elston, T. L. & Olwin, B. B. Isolation, culture, functional assays, and immunofluorescence of myofiber-associated satellite cells. Methods Mol. Biol. 1460, 141–162 (2016).
Motohashi, N., Asakura, Y. & Asakura, A. Isolation, culture, and transplantation of muscle satellite cells. J. Vis. Exp. 86, 50846 (2014).
Acharyya, S. et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 150, 165–178 (2012).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408 (2001).
Volodin, A., Kosti, I., Goldberg, A. L. & Cohen, S. Myofibril breakdown during atrophy is a delayed response requiring the transcription factor PAX4 and desmin depolymerization. Proc. Natl. Acad. Sci. USA 114, E1375–E1384 (2017).
Cosper, P. F. & Leinwand, L.A. Myosin heavy chain is not selectively decreased in murine cancer cachexia. Int. J. Cancer 130, 2722–2727 (2012).
Liberzon, A. et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 (2015).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Acknowledgements
We would like to thank A. Ferrando (Columbia University Medical Center, CUMC), G. Karsenty (CUMC), T. Oskarsson (Deutsches Krebsforschungszentrum/German Cancer Center), D. Guttridge (Ohio State University), U. Klein (Leeds, UK) and members of the Acharyya laboratory for helpful insights throughout the study. We thank J. Massagué (Memorial Sloan Kettering Cancer Center), J. Sage (Stanford University) and Y. Kang (Princeton University) for sharing cell lines. We would like to thank K. Macrenaris and R. Sponenburg from Northwestern University Quantitative Bio-element Imaging Center (QBIC), supported by NASA Ames Research Center NNA06CB93G, and Y. Zhang and K. Schey from Vanderbilt University Imaging Mass Spectrometry Center for invaluable help with single-fiber mass spectrometry analyses. We would like to thank L. Munoz and T. Waddell from the Department of Pathology and Cell Biology at New York Presbyterian Hospital Center for muscle collection during autopsies. University of Nebraska Medical Center Rapid Autopsy Program was supported by National Institutes of Health (NIH) P50CA127297, U01CA210240 and 5R50CA211462 (to P.M.G. and M.A.H.). Establishment of the congenic Balb/c Zip14-knockout mice was supported by DK080706 (to M.D.K.). Establishment of lung cancer models was supported by National Cancer Institute R00CA172697 (to S.A.). This work was supported by Institutional start-up funds from CUIMC to S.A. and by Herbert Irving Comprehensive Cancer Center’s 5P30CA013696-43 Cancer Center Support Grant-Inter-Programmatic Pilot Project to S.A. Schematic models were generated in part using Servier Medical Art, licensed under a Creative Commons Attribution (CC BY) 3.0 License, with further modifications in some cases.
Author information
Authors and Affiliations
Contributions
G.W., A.K.B., W.M., C.C. and S.A. designed and performed the experiments. G.W., A.K.B., W.M. and S.A. wrote the manuscript. P.M.G. and M.A.H. provided human muscle samples from the Rapid Autopsy Program (RAP)-Pancreas at the University of Nebraska Medical Center. R.J. assisted with antibody purification. K.T., A.B., and D.H. provided pathological characterization and oversaw muscle samples collection at CUMC. M.K. performed the bioinformatics analysis and was supervised by R.D. S.L.-P. provided biostatistics consultation. S.H., S.J., M.D.K. and T.F. provided the Zip14-knockout mice and reagents. A.K.B. and S.A. conceived the project. S.A. supervised all research. All authors read the manuscript and approved the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Tables 1–7
Rights and permissions
About this article
Cite this article
Wang, G., Biswas, A.K., Ma, W. et al. Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle. Nat Med 24, 770–781 (2018). https://doi.org/10.1038/s41591-018-0054-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-018-0054-2
This article is cited by
-
Cellular zinc metabolism and zinc signaling: from biological functions to diseases and therapeutic targets
Signal Transduction and Targeted Therapy (2024)
-
Activation of GPR81 by lactate drives tumour-induced cachexia
Nature Metabolism (2024)
-
Possible involvement of zinc transporter ZIP13 in myogenic differentiation
Scientific Reports (2024)
-
Transcriptional programming of translation by BCL6 controls skeletal muscle proteostasis
Nature Metabolism (2024)
-
Highlighting the idea of exerkines in the management of cancer patients with cachexia: novel insights and a critical review
BMC Cancer (2023)