Abstract
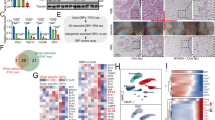
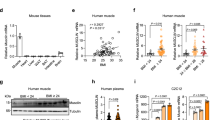
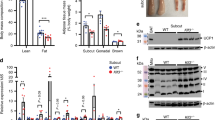
Beige adipocytes have recently been shown to regulate energy dissipation when activated and help organisms defend against hypothermia and obesity. Prior reports indicate that beige-like adipocytes exist in adult humans and that they may present novel opportunities to curb the global epidemic in obesity and metabolic illnesses. In an effort to identify unique features of activated beige adipocytes, we found that expression of the cholinergic receptor nicotinic alpha 2 subunit (Chrna2) was induced in subcutaneous fat during the activation of these cells and that acetylcholine-producing immune cells within this tissue regulated this signaling pathway via paracrine mechanisms. CHRNA2 functioned selectively in uncoupling protein 1 (Ucp1)-positive beige adipocytes, increasing thermogenesis through a cAMP- and protein kinase A-dependent pathway. Furthermore, this signaling via CHRNA2 was conserved and present in human subcutaneous adipocytes. Inactivation of Chrna2 in mice compromised the cold-induced thermogenic response selectively in subcutaneous fat and exacerbated high-fat diet-induced obesity and associated metabolic disorders, indicating that even partial loss of beige fat regulation in vivo had detrimental consequences. Our results reveal a beige-selective immune–adipose interaction mediated through CHRNA2 and identify a novel function of nicotinic acetylcholine receptors in energy metabolism. These findings may lead to identification of therapeutic targets to counteract human obesity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Sharp, L. Z. et al. Human BAT possesses molecular signatures that resemble beige or brite cells. PLoS One 7, e49452 (2012).
Lidell, M. E. et al. Evidence for two types of brown adipose tissue in humans. Nat. Med. 19, 631–634 (2013).
Shinoda, K. et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat. Med. 21, 389–394 (2015).
Lee, P. et al. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes 63, 3686–3698 (2014).
Hanssen, M. J. et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 21, 863–865 (2015).
Yoneshiro, T. et al. Recruited brown adipose tissue as an anti-obesity agent in humans. J. Clin. Invest. 123, 3404–3408 (2013).
Chondronikola, M. et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63, 4089–4099 (2014).
Kajimura, S., Spiegelman, B. M. & Seale, P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 22, 546–559 (2015).
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
Le Novère, N., Corringer, P. J. & Changeux, J. P. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J. Neurobiol. 53, 447–456 (2002).
Changeux, J. P. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat. Rev. Neurosci. 11, 389–401 (2010).
Ohno, H., Shinoda, K., Spiegelman, B. M. & Kajimura, S. PPAR-γ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 15, 395–404 (2012).
Qiang, L. et al. Brown remodeling of white adipose tissue by SIRT1-dependent deacetylation of PPAR-γ. Cell 150, 620–632 (2012).
Wilson-Fritch, L. et al. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J. Clin. Invest. 114, 1281–1289 (2004).
Bartesaghi, S. et al. Thermogenic activity of UCP1 in human white-fat-derived beige adipocytes. Mol. Endocrinol. 29, 130–139 (2015).
Elsen, M. et al. BMP4 and BMP7 induce the white-to-brown transition of primary human adipose stem cells. Am. J. Physiol. Cell Physiol. 306, C431–C440 (2014).
Kong, X. et al. IRF4 is a key thermogenic transcriptional partner of PGC-1α. Cell 158, 69–83 (2014).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Seale, P. et al. PRDM16 controls a brown fat–skeletal muscle switch. Nature 454, 961–967 (2008).
Svensson, P. A. et al. Characterization of brown adipose tissue in the human perirenal depot. Obesity (Silver Spring) 22, 1830–1837 (2014).
Nagano, G. et al. Activation of classical brown adipocytes in the adult human perirenal depot is highly correlated with PRDM16–EHMT1 complex expression. PLoS One 10, e0122584 (2015).
Ma, S. et al. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J. Mol. Cell Biol. 4, 88–96 (2012).
Tallini, Y. N. et al. BAC transgenic mice express enhanced green fluorescent protein in central and peripheral cholinergic neurons. Physiol. Genomics 27, 391–397 (2006).
Rosas-Ballina, M. et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 (2011).
Reardon, C. et al. Lymphocyte-derived ACh regulates local innate but not adaptive immunity. Proc. Natl. Acad. Sci. USA 110, 1410–1415 (2013).
Nguyen, K. D. et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480, 104–108 (2011).
Bachman, E. S. et al. β-AR signaling required for diet-induced thermogenesis and obesity resistance. Science 297, 843–845 (2002).
Fischer, K. et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat. Med. 23, 623–630 (2017).
Jiang, H., Ding, X., Cao, Y., Wang, H. & Zeng, W. Dense intra-adipose sympathetic arborizations are essential for cold-induced beiging of mouse white adipose tissue. Cell Metab. 26, 686–692 (2017).
Rowland, L. A., Bal, N. C., Kozak, L. P. & Periasamy, M. Uncoupling protein 1 and sarcolipin are required to maintain optimal thermogenesis, and loss of both systems compromises survival of mice under cold stress. J. Biol. Chem. 290, 12282–12289 (2015).
Bruton, J. D. et al. Increased fatigue resistance linked to Ca2+-stimulated mitochondrial biogenesis in muscle fibers of cold-acclimated mice. J. Physiol. (Lond.) 588, 4275–4288 (2010).
Cohen, P. et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous-to-visceral-fat switch. Cell 156, 304–316 (2014).
Sparks, L. M. et al. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 54, 1926–1933 (2005).
Brestoff, J. R. & Artis, D. Immune regulation of metabolic homeostasis in health and disease. Cell 161, 146–160 (2015).
Lumeng, C. N. & Saltiel, A. R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117 (2011).
Williamson, D. F. et al. Smoking cessation and severity of weight gain in a national cohort. N. Engl. J. Med. 324, 739–745 (1991).
Yoshida, T. et al. Nicotine induces uncoupling protein 1 in white adipose tissue of obese mice. Int. J. Obes. Relat. Metab. Disord. 23, 570–575 (1999).
Dodd, G. T. et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 160, 88–104 (2015).
Owen, B. M. et al. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure and weight loss. Cell Metab. 20, 670–677 (2014).
Luo, J. et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat. Protoc. 2, 1236–1247 (2007).
Song, P., Mabrouk, O. S., Hershey, N. D. & Kennedy, R. T. In vivo neurochemical monitoring using benzoyl chloride derivatization and liquid chromatography–mass spectrometry. Anal. Chem. 84, 412–419 (2012).
Goldschneider, I., Komschlies, K. L. & Greiner, D. L. Studies of thymocytopoiesis in rats and mice. I. Kinetics of appearance of thymocytes using a direct intrathymic adoptive transfer assay for thymocyte precursors. J. Exp. Med. 163, 1–17 (1986).
Galarraga, M. et al. Adiposoft: automated software for the analysis of white adipose tissue cellularity in histological sections. J. Lipid Res. 53, 2791–2796 (2012).
Weir, J. B. New methods for calculating metabolic rate with special reference to protein metabolism. 1949. Nutrition 6, 213–221 (1990).
Acknowledgements
We thank B. M. Spiegelman and J. D. Lin for their advice and discussion throughout this work, B. Lowell (Beth Israel Deaconess Medical Center) for the β-less mice, J. M. Gimble (Tulane University) for human subcutaneous adipose precursor cells and W. Rainey (University of Michigan) for human perirenal adipose precursor cells. The work was supported by the Human Frontier Science Program (grant no. RGY0082/14; J.W.), the Edward Mallinckrodt Jr. Foundation (J.W.), the American Diabetes Association (grant no. 1-18-IBS-281; J.W.), the US National Institutes of Health (grant no. R01DK107583 (J.W.), P30-DK020572 (J.W.), P30-DK089503 (J.W.), F31DK112625 (M.P.E.), R37EB003320 (R.T.K.) and DK046960 (R.T.K.), R01-AI091627 (I.M.) and T32DA007268 (A.G.Z.)) and a postdoctoral fellowship from the American Heart Association (17POST33060001; D.K.).
Author information
Authors and Affiliations
Contributions
H.J., H.Y. and J.W. designed the experiments and wrote the manuscript; H.J., H.Y., J.G., J.J., X.Q., E.P., D.K., M.P.E., A.G.Z., J.-S.C. and J.W. performed the experiments; and H.J., H.Y., J.G., E.P., A.G.Z., J.L., R.T.K., I.M., X.Z.S.X. and J.W. analyzed the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Figures and Tables
Supplementary Figures 1–12 and Supplementary Tables 1 and 2
Rights and permissions
About this article
Cite this article
Jun, H., Yu, H., Gong, J. et al. An immune-beige adipocyte communication via nicotinic acetylcholine receptor signaling. Nat Med 24, 814–822 (2018). https://doi.org/10.1038/s41591-018-0032-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-018-0032-8
This article is cited by
-
Macrophage and T cell networks in adipose tissue
Nature Reviews Endocrinology (2024)
-
Unraveling the complex roles of macrophages in obese adipose tissue: an overview
Frontiers of Medicine (2024)
-
Linker histone variant H1.2 is a brake on white adipose tissue browning
Nature Communications (2023)
-
Secreted EMC10 is upregulated in human obesity and its neutralizing antibody prevents diet-induced obesity in mice
Nature Communications (2022)
-
Olfactory perception of food abundance regulates dietary restriction-mediated longevity via a brain-to-gut signal
Nature Aging (2021)