Abstract
Cerebrovascular injury (CVI) is a common pathology caused by infections, injury, stroke, neurodegeneration and autoimmune disease. Rapid resolution of a CVI requires a coordinated innate immune response. In the present study, we sought mechanistic insights into how central nervous system-infiltrating monocytes program resident microglia to mediate angiogenesis and cerebrovascular repair after an intracerebral hemorrhage. In the penumbrae of human stroke brain lesions, we identified a subpopulation of microglia that express vascular endothelial growth factor A. These cells, termed ‘repair-associated microglia’ (RAMs), were also observed in a rodent model of CVI and coexpressed interleukin (IL)-6Ra. Cerebrovascular repair did not occur in IL-6 knockouts or in mice lacking microglial IL-6Ra expression and single-cell transcriptomic analyses revealed faulty RAM programming in the absence of IL-6 signaling. Infiltrating CCR2+ monocytes were the primary source of IL-6 after a CVI and were required to endow microglia with proliferative and proangiogenic properties. Faulty RAM programming in the absence of IL-6 or inflammatory monocytes resulted in poor cerebrovascular repair, neuronal destruction and sustained neurological deficits that were all restored via exogenous IL-6 administration. These data provide a molecular and cellular basis for how monocytes instruct microglia to repair damaged brain vasculature and promote functional recovery after injury.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of the present study are available from the corresponding author upon request. There are no restrictions on data availability. ScRNA-seq data are available in the National Center for Biotechnology Information Gene Expression Omnibus under accession no. GSE215312. The mouse genome database used in our scRNA-seq analysis was mm10 (https://www.ncbi.nlm.nih.gov/assembly/GCF_000001635.20). Source data are provided with this paper.
References
Parikh, N. S., Merkler, A. E. & Iadecola, C. Inflammation, autoimmunity, infection, and stroke: epidemiology and lessons from therapeutic intervention. Stroke 51, 711–718 (2020).
Kenney, K. et al. Cerebral vascular injury in traumatic brain injury. Exp. Neurol. 275, 353–366 (2016).
Schneider, J. A. & Bennett, D. A. Where vascular meets neurodegenerative disease. Stroke 41, S144–S146 (2010).
Badjatia, N. & Rosand, J. Intracerebral hemorrhage. Neurologist 11, 311–324 (2005).
Twitchell, T. E. The restoration of motor function following hemiplegia in man. Brain 74, 443–480 (1951).
Rossini, P. M., Calautti, C., Pauri, F. & Baron, J. C. Post-stroke plastic reorganisation in the adult brain. Lancet Neurol. 2, 493–502 (2003).
Furlan, M., Marchal, G., Viader, F., Derlon, J. M. & Baron, J. C. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann. Neurol. 40, 216–226 (1996).
Lalancette-Hebert, M., Gowing, G., Simard, A., Weng, Y. C. & Kriz, J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J. Neurosci. 27, 2596–2605 (2007).
Narantuya, D. et al. Human microglia transplanted in rat focal ischemia brain induce neuroprotection and behavioral improvement. PLoS ONE 5, e11746 (2010).
Mastorakos, P., Russo, M. V., Zhou, T., Johnson, K. & McGavern, D. B. Antimicrobial immunity impedes CNS vascular repair following brain injury. Nat. Immunol. 22, 1280–1293 (2021).
Schaeffer, S. & Iadecola, C. Revisiting the neurovascular unit. Nat. Neurosci. 24, 1198–1209 (2021).
Park, L. et al. Brain perivascular macrophages initiate the neurovascular dysfunction of Alzheimer abeta peptides. Circ. Res. 121, 258–269 (2017).
Nortley, R. et al. Amyloid beta oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 365, eaav9518 (2019).
Mastorakos, P. & McGavern, D. The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. 4, eaav0492 (2019).
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 (2005).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Roth, T. L. et al. Transcranial amelioration of inflammation and cell death after brain injury. Nature 505, 223–228 (2014).
Mastorakos, P. et al. Temporally distinct myeloid cell responses mediate damage and repair after cerebrovascular injury. Nat. Neurosci. 24, 245–258 (2021).
Lou, N. et al. Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc. Natl Acad. Sci. USA 113, 1074–1079 (2016).
Dimitrijevic, O. B., Stamatovic, S. M., Keep, R. F. & Andjelkovic, A. V. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke 38, 1345–1353 (2007).
Perego, C., Fumagalli, S. & De Simoni, M. G. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J. Neuroinflam. 8, 174 (2011).
Wattananit, S. et al. Monocyte-derived macrophages contribute to spontaneous long-term functional recovery after stroke in mice. J. Neurosci. 36, 4182–4195 (2016).
Prinz, M., Jung, S. & Priller, J. Microglia biology: one century of evolving concepts. Cell 179, 292–311 (2019).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290 e1217 (2017).
Nayak, D., Roth, T. L. & McGavern, D. B. Microglia development and function. Annu. Rev. Immunol. 32, 367–402 (2014).
Stratoulias, V., Venero, J. L., Tremblay, M. E. & Joseph, B. Microglial subtypes: diversity within the microglial community. EMBO J. 38, e101997 (2019).
Reitmeir, R. et al. Vascular endothelial growth factor induces contralesional corticobulbar plasticity and functional neurological recovery in the ischemic brain. Acta Neuropathol. 123, 273–284 (2012).
Kaiser, T. & Feng, G. Tmem119-EGFP and Tmem119-CreERT2 transgenic mice for labeling and manipulating microglia. eNeuro 6, ENEURO.0448–18.2019 (2019).
Ferrara, N. & Henzel, W. J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 161, 851–858 (1989).
Van Snick, J. Interleukin-6: an overview. Annu. Rev. Immunol. 8, 253–278 (1990).
Peters, M. et al. Combined interleukin 6 and soluble interleukin 6 receptor accelerates murine liver regeneration. Gastroenterology 119, 1663–1671 (2000).
Saederup, N. et al. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS ONE 5, e13693 (2010).
Serbina, N. V. & Pamer, E. G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317 (2006).
Tanaka, T., Narazaki, M. & Kishimoto, T. Interleukin (IL-6) Immunotherapy. Cold Spring Harb. Perspect. Biol. 10, a028456. (2018).
Chen, E. Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinf. 14, 128 (2013).
Marech, I. et al. Classical and non-classical proangiogenic factors as a target of antiangiogenic therapy in tumor microenvironment. Cancer Lett. 380, 216–226 (2016).
Hayashi, T., Noshita, N., Sugawara, T. & Chan, P. H. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J. Cereb. Blood Flow Metab. 23, 166–180 (2003).
Krupinski, J., Kaluza, J., Kumar, P., Kumar, S. & Wang, J. M. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 25, 1794–1798 (1994).
Kato, H., Kogure, K., Liu, X. H., Araki, T. & Itoyama, Y. Progressive expression of immunomolecules on activated microglia and invading leukocytes following focal cerebral ischemia in the rat. Brain Res. 734, 203–212 (1996).
Schroeter, M., Jander, S., Huitinga, I., Witte, O. W. & Stoll, G. Phagocytic response in photochemically induced infarction of rat cerebral cortex. The role of resident microglia. Stroke 28, 382–386 (1997).
Gliem, M. et al. Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann. Neurol. 71, 743–752 (2012).
Gliem, M. et al. Macrophage-derived osteopontin induces reactive astrocyte polarization and promotes re-establishment of the blood brain barrier after ischemic stroke. Glia 63, 2198–2207 (2015).
Hirano, T., Akira, S., Taga, T. & Kishimoto, T. Biological and clinical aspects of interleukin 6. Immunol. Today 11, 443–449 (1990).
Lin, Z. Q., Kondo, T., Ishida, Y., Takayasu, T. & Mukaida, N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J. Leukoc. Biol. 73, 713–721 (2003).
Atreya, R. & Neurath, M. F. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin. Rev. Allergy Immunol. 28, 187–196 (2005).
Gertz, K. et al. Essential role of interleukin-6 in post-stroke angiogenesis. Brain 135, 1964–1980 (2012).
Clark, W. M. et al. Lack of interleukin-6 expression is not protective against focal central nervous system ischemia. Stroke 31, 1715–1720 (2000).
Gronhoj, M. H., Clausen, B. H., Fenger, C. D., Lambertsen, K. L. & Finsen, B. Beneficial potential of intravenously administered IL-6 in improving outcome after murine experimental stroke. Brain Behav. Immun. 65, 296–311 (2017).
Li, Z. et al. M-CSF, IL-6, and TGF−beta promote generation of a new subset of tissue repair macrophage for traumatic brain injury recovery. Sci. Adv. 7, eabb6260 (2021).
Ormstad, H., Aass, H. C., Lund-Sorensen, N., Amthor, K. F. & Sandvik, L. Serum levels of cytokines and C-reactive protein in acute ischemic stroke patients, and their relationship to stroke lateralization, type, and infarct volume. J. Neurol. 258, 677–685 (2011).
Waje-Andreassen, U. et al. IL-6: an early marker for outcome in acute ischemic stroke. Acta Neurol. Scand. 111, 360–365 (2005).
Kitamura, Y. et al. Recovery of focal brain ischemia-induced behavioral dysfunction by intracerebroventricular injection of microglia. J. Pharm. Sci. 97, 289–293 (2005).
Koike, K. et al. Synergism of BSF-2/interleukin 6 and interleukin 3 on development of multipotential hemopoietic progenitors in serum-free culture. J. Exp. Med. 168, 879–890 (1988).
Bot, F. J., van Eijk, L., Broeders, L., Aarden, L. A. & Lowenberg, B. Interleukin-6 synergizes with M-CSF in the formation of macrophage colonies from purified human marrow progenitor cells. Blood 73, 435–437 (1989).
Uyttenhove, C., Coulie, P. G. & Van Snick, J. T cell growth and differentiation induced by interleukin-HP1/IL-6, the murine hybridoma/plasmacytoma growth factor. J. Exp. Med. 167, 1417–1427 (1988).
Hu, X. et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43, 3063–3070 (2012).
Askenase, M. H. et al. Longitudinal transcriptomics define the stages of myeloid activation in the living human brain after intracerebral hemorrhage. Sci. Immunol. 6, eabd6279 (2021).
Garbers, C. & Rose-John, S. Dissecting interleukin-6 classic- and trans-signaling in inflammation and cancer. Methods Mol. Biol. 1725, 127–140 (2018).
Willis, E. F. et al. Repopulating microglia promote brain repair in an IL-6-dependent manner. Cell 180, 833–846 e816 (2020).
Leibinger, M. et al. Transneuronal delivery of hyper-interleukin-6 enables functional recovery after severe spinal cord injury in mice. Nat. Commun. 12, 391 (2021).
Barrett, J. P. et al. Interferon-beta plays a detrimental role in experimental traumatic brain injury by enhancing neuroinflammation that drives chronic neurodegeneration. J. Neurosci. 40, 2357–2370 (2020).
Kopf, M. et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368, 339–342 (1994).
McFarland-Mancini, M. M. et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J. Immunol. 184, 7219–7228 (2010).
Jung, S. et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2019).
Levine, J. H. et al. Data-driven phenotypic dissection of AML reveals progenitor-like cells that correlate with prognosis. Cell 162, 184–197 (2015).
Acknowledgements
This research was supported by the intramural program at the NINDS, NIH. We thank A. Elkahloun and B. Sisay in the National Human Genome Research Institute Microarray core for their assistance with the scRNA-seq experiment. The present study utilized the computational resources of the NIH Biowulf HPC cluster (http://hpc.nih.gov).
Author information
Authors and Affiliations
Contributions
B.C. performed the design, data acquisition and analysis. K.J. conducted computational analyses of scRNA-seq data. D.M. performed the multiplex immunohistochemical staining on human brain sections. B.C. and D.B.M. wrote and edited the manuscript. D.B.M. supervised and directed the project, and participated in experimental design, data acquisition and analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: L. A. Dempsey, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
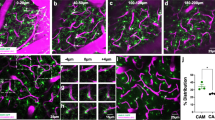
Extended Data Fig. 1 Identification of microglia in Tmem119-TdTo reporter mice.
a, Immunofluorescent staining for Tmem119 (green) and TdTo (red) of neocortex from a naïve Tmem119-TdTo mouse. Inset depicts an enlarged area of the image. b, Bar graph shows quantification of TdTo+ Tmem119+ microglia after tamoxifen treatment (n=5 mice). c, Histograms show TdTo expression on CD45+ CD11b+ cells harvested from the brains of uninjured (Ctrl) WT mice (n=4) as well as uninjured (n=4) and D6 post-CVI (n=4) Tmem119-TdTo mice. Each histogram represents concatenated samples for the entire group. Data are representative of two independent experiments. d, Histograms show TdTo expression in CD45+ CD11b+ myeloid cells isolated from the brain (n=8), meninges (n=6) and spleen (n=8) of uninjured and D6 post-CVI Tmem119-TdTo mice. The histogram represents a concatenation of both uninjured and injured samples. Data are representative of two independent experiments. Dashed lines in panels c and d denote the cut-off value for cells with TdTo positivity. Note that meningeal macrophages have more background fluorescence in this channel than splenic macrophages. e, Bar graph shows the absolute number of TdTo+ cells in the denoted tissues from uninjured Ctrl (blue) and D6 post-CVI (red) Tmem119-TdTo mice in panel c and d. (****P<0.0001; two-way ANOVA with Šídák’s multiple comparisons test). f, Gating strategy for flow cytometric analysis of leukocytes isolated from the brains of Tmem119-TdTo mice following CVI. g, Histogram shows TdTo expression in the indicated cell populations, identified using the gating strategy from panel f, from the brains of Tmem119-TdTo mice (n=5) at D6 post-CVI. h, Bar graph shows the absolute number of the denoted cell types from the brains of uninjured Ctrl (n=4) versus D6 post-CVI (n=4) Tmem119-TdTo mice. (****P<0.0001; two-way ANOVA with Šídák’s multiple comparisons test). Data are representative of two independent experiments. i, Representative TdTo expression (red) on a coronal brain section from a Tmem119-TdTo mouse (n=3) at D6 post CVI. Data are representative of two independent experiments. j, Pie chart depicts the percentage of VEGFA+ microglia (mean+SEM, red) among total CD11b+ CX3CR1+ TdTo+ microglia obtained from the punch biopsies of injured brains (n=4) at D6 post CVI described in Fig. 1i. k, A dimensionality reduction plot (top) generated using Cluster Explorer depicts three RAM clusters (shades of purple) identified in the brains of Tmem119-TdTo mice (n=4) at D6 post-CVI. Data are representative of two independent experiments. A representative dot plot (bottom) shows expression of VEGFA and IL-6Ra by the three RAM clusters identified in D6 Tmem119-TdTo mice. Arbitrary gating was used to analyze marker expression in panel l. Specifically, RAM were stratified based on low, intermediate, and high VEGFA and IL-6Ra co-expression: VEGFAlo IL-6Ralo (green), VEGFAint IL-6Raint (cyan), VEGFAhi IL-6Rahi (red). l, Histograms show expression of the indicated markers and FSC-A by the three RAM subpopulations identified in panel k. All graphs show mean ± SEM. Dots represent individual mice.
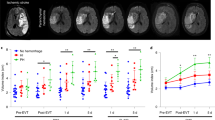
Extended Data Fig. 2 Mild versus severe CVI in mice and immunohistochemical staining of human brain tissue.
a, Dimensionality reduction plots generated using Cluster Explorer show RAM (blue), MHC IIhi RAM (red), non-RAM microglia (gray), and MDM (green) in Tmem119-TdTo mice on D6 post CVI. Plots show concatenated samples obtained from mild (n=4) and severe (n=4) WT CVI mice described in Fig. 2. The gating strategy for the different myeloid subsets is described in Fig. 2b-d. Data are representative of two independent experiments. b, Histograms show expression of the indicated markers by the different myeloid cell populations (a). c, Bar graph shows the percentage of MHCII+ VEGFA+ microglia in the brains of D6 Tmem119-TdTo mice described in Fig. 2a-d following mild versus severe CVI (****P<0.0001; two-tailed unpaired t test). d, Immunofluorescent staining of neocortical vasculature in uninjured Ctrl (n=8) as well as mild (n=6) and severe (n=7) CVI mice at day 10. Lectin (green) and Evans blue (red) were injected i.v. to visualize vessels and BBB disruption. e, Quantification showing numbers of extravasated dye among the groups of animals (Ctrl=8, mild CVI=6, and severe CVI=7). Data are combined from two independent experiments. (****P<0.0001; one-way ANOVA with Tukey’s test). All graphs show mean ± SEM. Dots represent individual mice. f, Multiplex immunostaining of human stroke (n=4) versus Ctrl (n=4) brain tissue (see Supplementary Table 1) showing expression of IBA1, CD31, glycophorin A (red blood cells, RBCs), VEGF-A, fibrinogen, and DAPI (cell nuclei). All images are comparably scaled. The magnified images in the bottom of each image depict VEGF-A (white) expression in IBA1+ myeloid cells (green).
Extended Data Fig. 3 Single cell RNA-seq analysis of WT vs Il6-/- CVI mice.
a-b, UMAPs show overlays of all single cell partitions for uninjured Ctrl versus WT D6 CVI (a) and WT CVI versus Il6-/- CVI mice (b). c-d, Bar graphs depict the number (c) and percentage (d) of cells corresponding to each mouse group for the 7 different partitions. e, UMAP inset shows an overlay of WT CVI versus Il6-/- CVI mice for all partitions. Top pathways are also shown based on gene set enrichment analysis of DEGs (Q<0.05) between injured WT versus Il6-/- mice in P1. Only statistically significant pathway terms are plotted on the graph (P<0.05; Fisher exact test), and dot size represents the odds ratio. f, Bar graph shows statistical significance (-log q value; blue) and the expression level (log2 fold change; red) for genes involved in angiogenesis, the top-rated signaling pathway from gene set enrichment analysis in (e). g, Heatmap shows the mean expression of genes associated with angiogenesis for injured WT and Il6-/- mice. The color legend (right) depicts the gene expression level. h-j, Top pathways are shown based on gene set enrichment analysis of DEGs (Q<0.05) between uninjured and injured WT mice in P1 C2 (h), 3 (i), and 4 (j). Only statistically significant pathway terms are plotted on the graphs (P<0.05; Fisher exact test), and dot size represents the odds ratio. k, Bar graph shows statistical significance (-log q value; blue) and the expression level (log2 fold change; red) for genes involved in angiogenesis for C2 vs C1, 3, and 4 within P1. l, Heatmap shows the mean expression of genes associated with angiogenesis in panel k.
Extended Data Fig. 4 Analysis of signaling pathways in microglia partitions identified by scRNA-seq.
a-b, UMAP insets show all partitions and the location of P2 (a) and 3 (b). Dot plots depict the top pathways in the respective partitions based on gene set enrichment analysis of DEGs (Q<0.05) between WT Ctrl and CVI mice. Dot size corresponds to the odds ratio. Only statistically significant pathways (P<0.05) are shown on the graph. Pathways are ordered based on the negative log of the P value multiplied by the corresponding Z score. The top ranked pathways are listed next to the UMAP insets. c, Heatmaps show mean expression levels of genes associated with IL-6/JAK/Stat3 signaling between WT and Il6-/- CVI mice for P1, 2, and 3. The color legend (right) depicts the gene expression level. d, UMAP plot shows differentiation trajectories for P1, 2, and 3 microglia. Individual dots represent single cells and the distance between two cells infers transcriptional similarity. Black traces represent trajectory branches. Each light grey circle denotes a different outcome of the trajectory. Black circles indicate branch nodes, from which cells can move to one of the denoted outcomes.
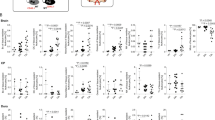
Extended Data Fig. 5 scRNA-seq analysis of macrophages and flow cytometric purity of sorted monocytes.
a, UMAP shows all partitions and the location of P4, which corresponds to macrophages. The top ranked pathway in P4 is noted above the UMAP. b, UMAPs depict expression of F13a1 and Tmem119 in all partitions. c, Pearson correlation-based clustered heatmap using DEGs (Q<0.05) between WT Ctrl and CVI mice in P4. The color legend reflects the Z score. d, Dot plot depicts the top pathways in P4 based on gene set enrichment analysis of DEGs (q<0.05) between WT and Il6-/- CVI mice. Dot size corresponds to the odds ratio. Only statistically significant pathways (P<0.05) are shown on the graph. Pathways are ordered based on the negative log of the P value multiplied by the corresponding Z score. e, Bar graph shows statistical significance (-log q value; blue) and the expression level (log2 fold change; red) for genes involved in TNFα signaling, the top-rated signaling pathway from gene set enrichment analysis in (d). Asterisks denote suppressor genes. f, Heatmap shows mean expression levels of genes in (e) for WT and Il6-/- CVI mice. The color legend (right) depicts the gene expression level. g, Gating strategy used for flow cytometric analysis of purified bone marrow-derived monocytes (CD11b+ Ly6C+ CX3CR1+ CD3- Siglec F- NK1.1- Ly6G- CD117- CD220-) from WT or Il6-/- mice that were adoptively transferred into Ccr2-/- recipients as described in Fig. 5d,e.
Extended Data Fig. 6 Quantification of cell proliferation and vascular leakage after CVI.
a-d, Representative confocal images from the neocortex stained for Iba1 (green) and EdU (white) (a) or CD31 (red) and EdU (white) (c). Bar graphs show the number of EdU+ Iba1+ cells (b) and EdU+ CD31+ cells (d) in uninjured WT Ctrl (n=9) versus D6 post-CVI WT (n=15), Ccr2-/- (n=7), and Il6-/- (n=7) mice at D6 post-CVI. Data are combined from two independent experiments. (**P<0.01, ***P<0.001, ****P<0.0001; one-way ANOVA with Tukey’s test). e-g, Representative confocal images from the neocortex stained for Iba1 (green), Tmem119-TdTo (red), CCR2-RFP (red), and EdU (white). Bar graphs show the number (f) and percentage (g) of EdU+ cells that are myeloid (green), microglia (red), and monocytes (blue) based on analysis of WT (n=8), Tmem119-TdTo (n=6), and CX3CR1gfp/+ CCR2rfp/+ (n=7) mice at D6 post-CVI. Data are combined from two independent experiments. (*P<0.05, ***P<0.001, ****P<0.0001; one-way ANOVA with Tukey’s test). h, Representative intravital TPM images captured through the thinned skull of uninjured WT Ctrl (n=8) versus CVI WT (n=8), Ccr2-/- (n=8), and Il6-/- (n=8) mice at D10 post-mild CVI. Tomato lectin (green) and Evans blue (red) were injected i.v. to visualize vasculature and dye extravasation. i, Bar graph depicts quantification of extravasated tomato lectin based on the dataset in (h). Data are combined from two independent experiments. (*P<0.05, ****P<0.0001; one-way ANOVA with Tukey’s test). j, Representative confocal images from the neocortex of uninjured WT Ctrl (n=7) versus D6 post-CVI WT (n=5), Ccr2-/- (n=6), and Il6-/- (n=6) mice at D10 post-CVI show i.v. injected tomato lectin (green) and Evans blue (red). k, Bar graph shows quantification of extravasated Evans blue (EB) based on the dataset shown in (j). Data are combined from two independent experiments. (*P<0.05, ***P<0.001; one-way ANOVA with Tukey’s test). All graphs show mean ± SEM. Dots represent individual mice.
Extended Data Fig. 7 Evaluation of Y-maze performance in uninjured mice and quantification of type 1 collagen deposition and Evans blue leakage after CVI.
a, Representative confocal images from the neocortex of WT (n=7), Ccr2-/- (n=7), and Il6-/- (n=7) mice at D10 post-CVI show staining for CD31 (green) and collagen type 1 (red). b, Bar graph shows quantification of collagen type 1 based on the dataset shown in (a). Data are combined from two independent experiments. (*P<0.05, **P<0.01; one-way ANOVA with Tukey’s test). c, Representative confocal images from the neocortex of WT (n=7), Ccr2-/- (n=7), and Il6-/- (n=7) mice at D10 post-CVI show staining for CD31 (green) and i.v. injected Evans blue (white). d, Bar graph shows quantification of extravasated Evans blue (EB) based on the dataset shown in (c). Data are combined from two independent experiments. (*P<0.05, ***P<0.001; one-way ANOVA with Tukey’s test). All graphs show mean ± SEM. Dots represent individual mice. e-f, Bar graphs demonstrate the total number of Y maze arm entries (e) and the triplicate ratio (f) for uninjured WT (n=6), Ccr2-/- (n=10), and Il6-/- (n=8) mice. Data are from a single experiment. All graphs show mean ± SEM (one-way ANOVA with Tukey’s test). Dots represent individual mice.
Supplementary information
Supplementary Video 1
Projection of microglial processes toward damaged blood vessels and debris after CVI. Part 1. A representative 3D time lapse captured through the thinned-skull window of a Tmem119-TdTo mouse (n = 4) shows the immediate microglial (green) response to a CVI. Blood vessels were labeled via intravenous injection of Tomato-lectin (red). White arrowheads denote microglial processes enveloping two different blood vessels, forming a tube-like structure. These vessels are Tomato-lectin negative because they are no longer flowing. Part 2. This enlarged view from Part 1 shows microglia acquiring Tomato-lectin-positive vascular debris (white arrowheads).
Supplementary Video 2
Monocyte extravasation into injured brain tissue after a CVI. Part 1. A representative 3D time lapse captured through the thinned-skull window of a B6 mouse at day 2 post-CVI shows extravasated Ly6C+ monocytes (red) migrating around an area of injured brain tissue. These monocytes were labeled via intravenous injection of anti-Ly6C-Alexa Fluor-647, which also labels vasculature (red). The white box demarcates the area enlarged in Part 2. Part 2. This enlarged region of the part 1 time lapse shows a Ly6C+ monocyte extravasating (white arrowheads) from a parenchymal blood vessel after CVI.
Supplementary Table 1
Table 1 Human brain tissue samples used for multiplex immunohistochemistry. Table 2 Antibodies for multiplex immunohistochemistry staining of human brain sections. Table 3 Antibodies for immunohistochemistry staining of mouse brain sections. Table 4 Surface marker antibodies for flow cytometry. Table 5 CD4 antibodies used for single color controls. Tables 6–26 ScRNA-seq group comparisons and statistical analyses
Supplementary Table 2
Statistical analysis for graphs in Figs. 1d,e,g,h,j,k,l, 2b–d,f,g,i, 3b,e,g,h, j,k, 5b–e,g,h,j,l,m, 6c,d,f,g,i,k,l,m and 7b,c,e,f,h,i,j. and Extended Data Figs. 1e,h, 2c,e, 6b,d,f,g,i,k and 7b,d,e,f.
Source data
Source Data Fig. 1
Source data for graphs in Fig. 1d,e,g,h,j,k,l.
Source Data Fig. 2
Source data for graphs in Fig. 2b–d,f,g,i.
Source Data Fig. 3
Source data for graphs in Fig. 3b,e,g,h, j,k.
Source Data Fig. 5
Source data for graphs in Fig. 5b–e,g,h,j,l,m
Source Data Fig. 6
Source data for graphs in Fig. 6c,d,f,g,i,k,l,m.
Source Data Fig. 7
Source data for graphs in Fig. 7b,c,e,f,h,i,j.
Source Data Extended Data Fig. 1
Source data for graphs in Extended Data Fig. 1e,h.
Source Data Extended Data Fig. 2
Source data for graphs in Extended Data Fig. 2c,e.
Source Data Extended Data Fig. 6
Source data for graphs in Extended Data Fig. 6b,d,f,g,i,k.
Source Data Extended Data Fig. 7
Source data for graphs in Extended Data Fig. 7b,d,e,f.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choi, BR., Johnson, K.R., Maric, D. et al. Monocyte-derived IL-6 programs microglia to rebuild damaged brain vasculature. Nat Immunol 24, 1110–1123 (2023). https://doi.org/10.1038/s41590-023-01521-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-023-01521-1