Abstract
Allergic diseases are a major global health issue. Interleukin (IL)-9-producing helper T (TH9) cells promote allergic inflammation, yet TH9 cell effector functions are incompletely understood because their lineage instability makes them challenging to study. Here we found that resting TH9 cells produced IL-9 independently of T cell receptor (TCR) restimulation, due to STAT5- and STAT6-dependent bystander activation. This mechanism was seen in circulating cells from allergic patients and was restricted to recently activated cells. STAT5-dependent Il9/IL9 regulatory elements underwent remodeling over time, inactivating the locus. A broader ‘allergic TH9’ transcriptomic and epigenomic program was also unstable. In vivo, TH9 cells induced airway inflammation via TCR-independent, STAT-dependent mechanisms. In allergic patients, TH9 cell expansion was associated with responsiveness to JAK inhibitors. These findings suggest that TH9 cell instability is a negative checkpoint on bystander activation that breaks down in allergy and that JAK inhibitors should be considered for allergic patients with TH9 cell expansion.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Raw and analyzed data are available at the Gene Expression Omnibus under accession no. GSE222910. Source data are provided with this paper.
Change history
11 October 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41590-023-01674-z
References
American Academy of Allergy Asthma & Immunology. Allergy Statistics https://www.aaaai.org/About/News/For-Media/Allergy-Statistics (2021).
Angkasekwinai, P. & Dong, C. IL-9-producing T cells: potential players in allergy and cancer. Nat. Rev. Immunol. 21, 37–48 (2021).
Shimbara, A. et al. IL-9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. J. Allergy Clin. Immunol. 105, 108–115 (2000).
Brough, H. A. et al. IL-9 is a key component of memory TH cell peanut-specific responses from children with peanut allergy. J. Allergy Clin. Immunol. 134, 1329–1338 (2014).
Abdelilah, S. et al. Functional expression of IL-9 receptor by human neutrophils from asthmatic donors: role in IL-8 release. J. Immunol. 166, 2768–2774 (2001).
Liu, J. et al. IL-9 regulates allergen-specific Th1 responses in allergic contact dermatitis. J. Invest. Dermatol. 134, 1903–1911 (2014).
Yao, W. et al. Interleukin-9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity 38, 360–372 (2013).
Wilhelm, C. et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat. Immunol. 12, 1071–1077 (2011).
Licona-Limon, P. et al. Th9 cells drive host immunity against gastrointestinal worm infection. Immunity 39, 744–757 (2013).
Kaplan, M. H., Hufford, M. M. & Olson, M. R. The development and in vivo function of T helper 9 cells. Nat. Rev. Immunol. 15, 295–307 (2015).
Gerlach, K. et al. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat. Immunol. 15, 676–686 (2014).
Xue, G., Jin, G., Fang, J. & Lu, Y. IL-4 together with IL-1β induces antitumor Th9 cell differentiation in the absence of TGF-β signaling. Nat. Commun. 10, 1376 (2019).
Nakatsukasa, H. et al. The DNA-binding inhibitor Id3 regulates IL-9 production in CD4(+) T cells. Nat. Immunol. 16, 1077–1084 (2015).
Schlapbach, C. et al. Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci. Transl. Med. 6, 219ra218 (2014).
Seumois, G. et al. Single-cell transcriptomic analysis of allergen-specific T cells in allergy and asthma. Sci. Immunol. 5, eaba6087 (2020).
Schwartz, D. M. et al. Retinoic acid receptor α represses a Th9 transcriptional and epigenomic program to reduce allergic pathology. Immunity 50, 106–120 (2019).
Kaplan, M. H. The transcription factor network in Th9 cells. Semin. Immunopathol. 39, 11–20 (2017).
Ulrich, B. J. et al. Allergic airway recall responses require IL-9 from resident memory CD4(+) T cells. Sci. Immunol. 7, eabg9296 (2022).
Lee, W. H. et al. BATF3 is sufficient for the induction of Il9 expression and can compensate for BATF during Th9 cell differentiation. Exp. Mol. Med. 51, 1–12 (2019).
Gomez-Rodriguez, J. et al. ITK is required for Th9 differentiation via TCR-mediated induction of IL-2 and IRF4. Nat. Commun. 7, 10857 (2016).
Richard, A. C. et al. The TNF-family ligand TL1A and its receptor DR3 promote T cell-mediated allergic immunopathology by enhancing differentiation and pathogenicity of IL-9-producing T cells. J. Immunol. 194, 3567–3582 (2015).
Xiao, X. et al. OX40 signaling favors the induction of T(H)9 cells and airway inflammation. Nat. Immunol. 13, 981–990 (2012).
Micosse, C. et al. Human ‘TH9’ cells are a subpopulation of PPAR-γ(+) TH2 cells. Sci. Immunol. 4, eaat5943 (2019).
Liao, W. et al. Opposing actions of IL-2 and IL-21 on Th9 differentiation correlate with their differential regulation of BCL6 expression. Proc. Natl Acad. Sci. USA 111, 3508–3513 (2014).
Fu, Y. et al. STAT5 promotes accessibility and is required for BATF-mediated plasticity at the Il9 locus. Nat. Commun. 11, 4882 (2020).
Harusato, A. et al. IL-36γ signaling controls the induced regulatory T cell–Th9 cell balance via NFκB activation and STAT transcription factors. Mucosal Immunol. 10, 1455–1467 (2017).
Dardalhon, V. et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+IL-10+ Foxp3(−) effector T cells. Nat. Immunol. 9, 1347–1355 (2008).
Olson, M. R. et al. Paracrine IL-2 is required for optimal type 2 effector cytokine production. J. Immunol. 198, 4352–4359 (2017).
Harnett, W. & Harnett, M. M. Helminth-derived immunomodulators: can understanding the worm produce the pill. Nat. Rev. Immunol. 10, 278–284 (2010).
Guo, L. et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc. Natl Acad. Sci. USA 106, 13463–13468 (2009).
Canaria, D. A. et al. IL-1β promotes IL-9-producing Th cell differentiation in IL-2-limiting conditions through the inhibition of BCL6. Front. Immunol. 13, 1032618 (2022).
Luo, Y. et al. JAK–STAT signaling in human disease: from genetic syndromes to clinical inhibition. J. Allergy Clin. Immunol. 148, 911–925 (2021).
Wang, Y. et al. Germinal-center development of memory B cells driven by IL-9 from follicular helper T cells. Nat. Immunol. 18, 921–930 (2017).
Salerno, F. et al. Translational repression of pre-formed cytokine-encoding mRNA prevents chronic activation of memory T cells. Nat. Immunol. 19, 828–837 (2018).
Zhang, Y. et al. Human TH9 differentiation is dependent on signal transducer and activator of transcription (STAT) 3 to restrain STAT1-mediated inhibition. J. Allergy Clin. Immunol. 143, 1108–1118 (2019).
Canaria, D. A. et al. STAT5 represses a STAT3-independent Th17-like program during Th9 cell differentiation. J. Immunol. 207, 1265–1274 (2021).
Olson, M. R., Verdan, F. F., Hufford, M. M., Dent, A. L. & Kaplan, M. H. STAT3 impairs STAT5 activation in the development of IL-9-secreting T cells. J. Immunol. 196, 3297–3304 (2016).
Shih, H. Y. et al. Developmental acquisition of regulomes underlies innate lymphoid cell functionality. Cell 165, 1120–1133 (2016).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Abdul Qayum, A. et al. The Il9 CNS-25 regulatory element controls mast cell and basophil IL-9 production. J. Immunol. 203, 1111–1121 (2019).
Xiao, X. et al. Guidance of super-enhancers in regulation of IL-9 induction and airway inflammation. J. Exp. Med. 215, 559–574 (2018).
Koh, B. et al. A conserved enhancer regulates Il9 expression in multiple lineages. Nat. Commun. 9, 4803 (2018).
Guo, L. et al. Innate immunological function of TH2 cells in vivo. Nat. Immunol. 16, 1051–1059 (2015).
Pavel, A. B. et al. Oral janus kinase/SYK inhibition (ASN002) suppresses inflammation and improves epidermal barrier markers in patients with atopic dermatitis. J. Allergy Clin. Immunol. 144, 1011–1024 (2019).
Lu, Y. et al. Th9 cells represent a unique subset of CD4(+) T cells endowed with the ability to eradicate advanced tumors. Cancer Cell 33, 1048–1060 (2018).
Chopp, L., Redmond, C., O’Shea, J. J. & Schwartz, D. M. From thymus to tissues and tumors: a review of T-cell biology. J. Allergy Clin. Immunol. https://doi.org/10.1016/j.jaci.2022.10.011 (2022).
Joyce, J. A. & Fearon, D. T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 (2015).
Elyaman, W. & Khoury, S. J. Th9 cells in the pathogenesis of EAE and multiple sclerosis. Semin. Immunopathol. 39, 79–87 (2017).
Caucheteux, S. M. et al. IL-1β enhances inflammatory TH2 differentiation. J. Allergy Clin. Immunol. 138, 898–901 (2016).
Ansel, K. M., Djuretic, I., Tanasa, B. & Rao, A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu. Rev. Immunol. 24, 607–656 (2006).
Herndler-Brandstetter, D. et al. KLRG1(+) effector CD8(+) T cells lose KLRG1, differentiate into ALL memory T cell lineages, and convey enhanced protective immunity. Immunity 48, 716–729 (2018).
Zhu, J. et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat. Immunol. 5, 1157–1165 (2004).
Gurram, R. K. et al. Crosstalk between ILC2s and Th2 cells varies among mouse models. Cell Rep. 42, 112073 (2023).
Liu, P., Jenkins, N. A. & Copeland, N. G. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 13, 476–484 (2003).
Corces, M. R. et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat. Methods 14, 959–962 (2017).
Buenrostro, J. D., Wu, B., Chang, H. Y. & Greenleaf, W. J. ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 109, 21.29.1–21.29.9 (2015).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Mootha, V. K. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Davis, C. A. et al. The encyclopedia of DNA elements (ENCODE): data portal update. Nucleic Acids Res. 46, D794–D801 (2018).
Acknowledgements
The authors thank R. Flavell and P. Licona-Limon for generously providing INFER and Klrg1Cre mice. We thank F. Petermann, Y. Baumer and S. Muallem for their thoughtful review of and comments on the manuscript. We acknowledge staff at the NIAID flow core for their assistance with flow sorting and analysis and the NIAMS sequencing core for assistance with sequencing and analysis. We thank J. Reilley and J. Cannons for assistance with sample retrieval. The study was funded by the Intramural Research Programs of NIAID and NIAMS. The relevant grants are ZIA-AI001251 (to D.M.S.), ZIA-AI001202 (to P.A.F.-G.), ZIA-AI001098 (to J.D.M.), ZIA-AI001240 (to P.S.) and ZIC-AR041181 (to F.M.).
Author information
Authors and Affiliations
Contributions
F.M. and J.G.-R. contributed equally to this manuscript. A.S., F.M., J.G.-R., Z.K., M.K., M.S., G.F., C.W., T.H., E.V., J.C., D.M.S. and C.F.Q. performed experiments and analyzed data. K.M. and J.D.M. provided patient samples. R.G. and J.Z. provided proprietary reagents. A.S. and D.M.S. authored the manuscript. J.D.M., P.S., P.F.G. and D.M.S. provided supervision.
Corresponding author
Ethics declarations
Competing interests
J.G.-R. is currently employed by TCR Therapeutics. F.M. has been recruited by AstraZeneca and was very briefly employed by Innovent Biologics. All other authors have no competing interests.
Peer review
Peer review information
Nature Immunology thanks Lionel Apetoh, Paula Licona-Limon and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: L. A. Dempsey, in collaboration with the Nature Immunology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Corresponds to Fig. 1.
a–e. Naïve CD4+ T cells from HVs were activated for 5 d with αCD3, αCD28, IL-2 and subset-promoting cytokines and antibodies. After 5 d, αCD3/αCD28 were withdrawn, and cells were cultured with IL-2 and subset-promoting cytokines and antibodies. Pooled results show production of IFN-γ (a), n = 9 (Th1, Th2) or 10 (Th9); IL-4 (b), n = 8 (Th1), 9 (Th2), or 4 (Th9); IL-13 (c) n = 8 (Th1), 9 (Th2), or 4 (Th9); IL-9 (d) n = 9 (Th1, Th2), or 10 (Th9); and IL-2 (e) n = 9 (Th1, Th2), or 10 (Th9), with or without PMA and Ionomycin (P/I). *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.0001, Wilcoxon. f. Bar graphs show % IL-9 positive cells differentiated to d8 Th9 as above and restimulated with vehicle versus plate-bound αCD3 at escalating doses (0.1, 0.5, 1, 5, 10, 50, 100 μg/mL), or P/I. *p < 0.05, **p < 0.01, ratio paired t-test (n = 3) g–k. Naïve CD4+ T cells from WT C57BL/6 mice were activated for 3 d with αCD3, αCD28, IL-2, and subset-promoting cytokines and antibodies. After 3 d, αCD3/αCD28 were withdrawn, and cells were cultured with IL-2 and subset-promoting cytokines and antibodies. Pooled results show production of IFN-γ (f), n = 6 (Th1, Th2) or 3 (Th9); IL-4 (g), n = 6 (Th1, Th2) or 5 (Th9); IL-13 (h), n = 6 (Th1), 7 (Th2), or 5 (Th9); IL-9 (i), n = 6 (Th1), 7 (Th2), or 10 (Th9); and IL-2 (j), n = 4 (Th1), 5 (Th2), or 3 (Th9), with or without P/I. *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.0001, Wilcoxon. l. Bar graphs show % IL-9+ cells of resting (d8) human Th9 cells restimulated with Th9 supernatants and vehicle, αIL-2 (20 μg/mL), αIL-4 (20 μg/mL), or αIL-1β (20 μg/mL). *p < 0.05, **p < 0.01, paired t-test versus Th9 supernatants (n = 3). For all line/bar graphs, error bars show ± s.e.m. Box plots show all data points (min to max, lines at median). All statistical tests are 2-sided.
Extended Data Fig. 2 Corresponds to Fig. 2.
a. Graph shows % IL-9+ of d8 human Th9 cells restimulated with vehicle (n = 7), IL-1β (n = 3), IL-18 (n = 4), IL-33 (n = 4), IL-36α (n = 7), or IL-36γ (n = 7). b, c. Timelines show experimental design; graphs shows % IL-9+ (n = 6) for human non-restimulated T cells differentiated as in Fig. 1a, with STAT5 inhibitor or STAT6 inhibitor from d0-5 (b) or d5-8 (c). d–f. Bar graph shows concentration (pg/mL, ELISA) of IL-2 (d), IL-4 (e), and IL-9 (f) in supernatants of human in vitro differentiated Th1- or Th9 (d5), or Th2 (d14) cells, after 48 h restimulation. g, h. d8 human Th9 cells were restimulated with escalating doses of IL-2 (b) (2, 20, or 200 ng/mL) or IL-4 (c) (6, 60, or 500 ng/mL). *p < 0.05, paired t-test (n = 3). i, j. Representative flow plots (i) and bar graph (j) show % IL-9+ cells in resting (d4) murine Th9 cells restimulated with IL-2 + IL-4 (n = 10), **p < 0.01, Wilcoxon test. k. Bar graph shows pooled results d8 human Th9 cells restimulated with vehicle (n = 9), IL-6 (n = 3), IL-7 (n = 5), IL-12 (n = 3), IL-21 (n = 3), or TSLP (n = 4). l, m. Bar graphs show pooled (n = 6) mean fluorescence intensity (MFI) of pSTAT5 (l) and pSTAT6 (m) in d8 human IL-9+ and IL-9− Th9 cells restimulated with IL-2, IL-4, or IL-2 + IL-4. *p < 0.05, **p < 0.01, Wilcoxon. n. Bar graphs show pooled (n = 3) % IL-9+ d8 human Th9 cells stimulated with IL-2 and IL-4 in the presence of STAT5 or STAT6 inhibitor. *p < 0.05, paired t-test. For all line/bar graphs, error bars show ± s.e.m. Box plots show all data points (min to max, lines at median). All statistical tests are 2-sided.
Extended Data Fig. 3 Corresponds to Fig. 3.
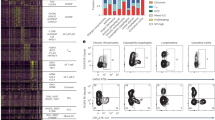
a, b. Line graph shows % IL-9+ cells of human Th9 differentiated as in Fig. 1a. Cells were restimulated with vehicle, IL-2, IL-4, or IL-2 + IL-4 (a, n = 5); b. on d5 (n = 14), d8/d11 (n = 17), d15 (n = 16), and d20 (n = 11), cells were restimulated with PMA + ionomycin (P/I) or P/I + IL-2 + IL-4. c, d. Line graph shows % IL-9+ cells of murine Th9 differentiated as in Fig. 1d. Cells were restimulated with vehicle, IL-2, IL-4, or IL-2 + IL-4 (c, n = 4). d. On d3 (n = 12), d4 (n = 11), d5 (n = 9), d6 (n = 3), d7 (n = 3), and d8 (n = 6), cells were restimulated P/I or P/I + IL-2 + IL-4. e, f. Representative flow plots (e) and graphs (f) show % IL-9+ of circulating Th9 cells stimulated with P/I or P/I + IL-2 + IL-4, from HV (n = 5) or atopic patients (AD, n = 14). g. Line graphs shows pooled results (n = 5) for % IL-9+ cells from IL-9 reporter (INFER) mice, sorted on d3 and maintained as in Fig. 1d. Cells were restimulated with vehicle, IL-2 + IL-4, P/I or P/I + IL-2 + IL-4. h. Bar graphs show viability (n = 4) for Th9 cells. For all experiments: *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001, paired t-test. i. Venn diagram shows generation of ‘allergic Th9’ cassette using: HDM-reactive circulating Th9 cells from allergic patients1, Th9 clones from healthy subjects2, pulmonary Th9 cells from IL-9 reporter mice, and in vitro differentiated Th9 cells3. Genes differentially expressed >1 dataset were selected. j. Bar graph shows pathway enrichment scores and FDRs (DAVID) for the ‘allergic Th9’ cassette. k. Scatterplot shows enrichment score (GSEA) and FDR for correlation (Pearson) of STAT1, STAT3, STAT4, STAT5, and STAT6 target genes with murine Il9 expression and human IL9 expression. For all line/bar graphs, error bars show ± s.e.m. Box plots show all data points (min to max, lines at median). All statistical tests are two-sided.
Extended Data Fig. 4 Corresponds to Fig. 4.
a, b. Genome tracks show poised enhancer (H3K4m1, red), active promoter (H3K4m3, blue), active enhancer (H3K27Ac, purple) marks, and accessibility (ATAC, green) of the murine Th2 (a, Il4−Il13-Rad50-Il5) and Gzmb loci at different time points during Th9 differentiation and resting. c, d. ATAC-seq tracks show the human extended IL9 locus including the promoter (Il9p), downstream enhancer (DS) and upstream enhancers 1–4 (E1-E4), in naïve T cells, Th1 cells (d5), and Th9 cells (d5) (c), and in ex vivo naïve, Th1, Th2, and Th17 cells from ENCODE (d). e, f. Genome tracks show poised enhancer (H3K4m1, red), active promoter (H3K4m3, blue), active enhancer (H3K27Ac, purple) marks, and accessibility (ATAC, green) of the human Th2 (e, IL4-IL13-RAD50-IL5) and GZMB loci at different time points during Th9 differentiation and resting. g. Heatmap shows Pearson correlation of Il9/IL9 expression, ATAC-seq tag density, H3K4me1 tag density, H3K4me3 tag density, and H3K27Ac tag density with those of Il4/IL4, Il13/IL13, Rad50/RAD50, and Il5/IL5.
Extended Data Fig. 5 Corresponds to Fig. 5.
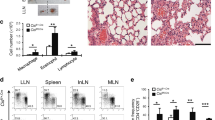
a–c. Pooled counts of pulmonary CD45+TCRβ+CD4+CD44+IL-13+ cells (a, n = 8, PBS; n = 13, papain), CD45+TCRβ+CD4+CD44+IL-17A+ cells (b, n = 3, PBS; n = 6, papain), and CD45+TCRβ+CD4+CD44+IL-2+ cells (c, n = 8, PBS; n = 13, papain) from mice treated with isotype versus αMHC2 between papain sensitization and challenge. d. Th9 cells were differentiated in the presence of vehicle versus BMS-509744 (Itk inhibitor; 0.5 μM, 1 μM, 1.5 μM) and restimulated with αCD3 + αCD28 (n = 3) e-f. Bar graphs (n = 7) show %IL-9+ (e) of Th9 and %IL-4+ (f) of Th2 cells restimulated with αCD3 + αCD28 and vehicle versus BMS-509744 (0.5 μM, 1 μM), *P < 0.05 paired t-test. g. Design of inducible Itk-deleted mouse. Itkflox/flox were crossed ERT-Cre mice to generate ItkERT mice. h. Representative flow plot and bar graph show %IL-9+ cells of Th9 cells from TAM-treated (Itk-deleted, n = 3) versus TAM-untreated (n = 5) mice, *P < 0.05 Mann–Whitney U-test. i. Timeline shows model of papain-induced airway inflammation with Itk deletion. Mice were treated with vehicle versus tamoxifen between sensitization and challenge. j. Gel shows that injection of tamoxifen results in deletion of WT Itk gene in mice from (i). k–n. Representative periodic acid-Schiff (PAS) stained images (k), pooled histology scores (l, n = 3, PBS; n = 5, papain), pulmonary CD45+TCRβ+CD4+CD44+IL-9+ (Th9) (m, n = 5, PBS; n = 10, papain), and pulmonary CD45+TCRβ+CD4+CD44+IL-13+ (Th2) cell counts (n, n = 3, PBS; n = 5, papain), from Itkf/f treated with vehicle versus tamoxifen as in (i). o–r. Pooled counts of pulmonary CD45+CD4- IL-9+ cells (o, n = 5, PBS; n = 8, papain), CD45+TCRβ+CD4+CD44+IL-13+ cells (Th2) (p, n = 5, PBS; n = 8, papain), CD45+TCRβ+CD4+CD44+IL-17A+ cells (Th17) (q, n = 4, PBS; n = 7, papain), and CD45+TCRβ+CD4+CD44+IL-2+ cells (r n = 4, PBS; n = 7, papain) ILC2-deficient mice treated with isotype versus αMHC2 between papain sensitization and challenge. For all in vivo experiments, *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001, Mann–Whitney U-test. For all line/bar graphs, error bars show ± s.e.m. Box plots show all data points (min to max, lines at median). All statistical tests were two-sided.
Extended Data Fig. 6 Corresponds to Fig. 6.
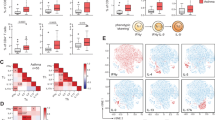
a. Timeline shows Th9 adoptive transfer-induced airway inflammation. Ovalbumin (OVA)-specific OT-ii Th9 cells were differentiated in vitro, sorted for IL-9+ cells, and adoptively transferred. Recipient mice were treated with IL-2 and IL-4 intratracheally and intranasally. b. Timeline shows papain-induced airway inflammation with tofacitinib; mice were treated with vehicle versus tofacitinib between sensitization and challenge. c–e. Pooled counts of pulmonary CD45+TCRβ+CD4+CD44+IL-13+ (c), CD45+TCRβ+CD4+CD44+IL-17A+ (d), and CD45+TCRβ+CD4+CD44+IL-2+ cells (e) mice treated with vehicle versus tofacitinib between sensitization and challenge. n = 6 (PBS/PBS), 5 (PBS/tofa), 10 (papain/MC and papain/tofa) f. Timeline shows papain-induced airway inflammation with tofacitinib and αIL-9. Mice were treated as in (b) but also received isotype or αIL-9 every other day. g–k. Pooled counts of pulmonary CD45+TCRβ+CD4+CD44+IL-9+ (g), CD45+TCRβ+CD4+CD44+IL-13+ (h), and live CD45+TCRβ+CD4+CD44+IL-4+ cells (i), representative periodic acid-Schiff (PAS) stained images (j) and pooled histology scores (k) of mice treated as in Extended Data Figure 6f; n = 5 (PBS/isotype/MC), 8 (papain/isotype/MC), and 10 (papain/αIL-9/MC and papain/αIL-9/tofa). For all in vivo experiments, *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001, Mann–Whitney U-test. l-o. Line graphs show expression over 96 h of IL9 (l), STAT5A (m), STAT5B (n), and STAT6 (o), in skin of nickel-allergic patients exposed to nickel over 96 h (n = 7) p. Graph shows normalized enrichment scores and FDR (GSEA) for expression of ‘allergic Th9’ cassette, STAT5-induced genes, and STAT6-induced genes in 7h-nickel-exposed, 48h-nickel-exposed, and 96h-nickel-exposed skin relative to pre-exposed/unexposed skin. For all line/bar graphs, error bars show ± s.e.m. Box plots show all data points (min to max, lines at median). All statistical tests are two-sided.
Extended Data Fig. 7 Reporting of gating strategy and validation for murine cell studies.
a–f. Representative flow cytometric plots show gating strategies for naïve T cell sorting (a), Th9 sorting for transfer experiments (b), for in vitro differentiated Th9, Th1, and Th2 cells shown in Figs. 1–3 and Extended Data Figs. 1–3(c), for in vivo memory T helper subsets in Figs. 5, 6 and Extended Data Figs. 5, 6(d, e), and for adoptively transferred CD45.1+ Th9 cells analyzed in murine lung 2 days after transfer (f). g. Timeline shows protocol for validation of MHC2 blocking antibody in vivo. Mice were sensitized to ovalbumin (OVA) intraperitoneally on d0 and d7, then challenged on d13 and d14. MHC blocking antibody was administered with each dose of OVA. Bar graphs show results of MHC2 validation experiment. Blockade of MHC2 reduced histology scores, BAL Eosinophil counts, and lung-infiltrating IL-4+ and IL-13+ (Th2) cell counts. *P < 0.05, **P < 0.01, Mann–Whitney U-test. All statistical tests are two-sided.
Supplementary information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed DNA electrophoresis gel.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Methods
Validation material and dilutions for antibodies.
Rights and permissions
About this article
Cite this article
Son, A., Meylan, F., Gomez-Rodriguez, J. et al. Dynamic chromatin accessibility licenses STAT5- and STAT6-dependent innate-like function of TH9 cells to promote allergic inflammation. Nat Immunol 24, 1036–1048 (2023). https://doi.org/10.1038/s41590-023-01501-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-023-01501-5
This article is cited by
-
JAKing up IL-9 expression in TH9 cells
Nature Immunology (2023)
-
JAKs drive innate-like TH9 cell activation in allergy
Nature Reviews Immunology (2023)