Abstract
Immunoglobulin A (IgA) secretion by plasma cells, terminally differentiated B cells residing in the intestinal lamina propria, assures microbiome homeostasis and protects the host against enteric infections. Exposure to diet-derived and commensal-derived signals provides immune cells with organizing cues that instruct their effector function and dynamically shape intestinal immune responses at the mucosal barrier. Recent data have described metabolic and microbial inputs controlling T cell and innate lymphoid cell activation in the gut; however, whether IgA-secreting lamina propria plasma cells are tuned by local stimuli is completely unknown. Although antibody secretion is considered to be imprinted during B cell differentiation and therefore largely unaffected by environmental changes, a rapid modulation of IgA levels in response to intestinal fluctuations might be beneficial to the host. In the present study, we showed that dietary cholesterol absorption and commensal recognition by duodenal intestinal epithelial cells lead to the production of oxysterols, evolutionarily conserved lipids with immunomodulatory functions. Using conditional cholesterol 25-hydroxylase deleter mouse line we demonstrated that 7α,25-dihydroxycholesterol from epithelial cells is critical to restrain IgA secretion against commensal- and pathogen-derived antigens in the gut. Intestinal plasma cells sense oxysterols via the chemoattractant receptor GPR183 and couple their tissue positioning with IgA secretion. Our findings revealed a new mechanism linking dietary cholesterol and humoral immune responses centered around plasma cell localization for efficient mucosal protection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The 16S rRNA data have been deposited on the NCBI Sequence Read Archive (SRA) (accession no. PRJEB58116). Source data are provided with this paper.
Code availability
Code to reproduce analysis has been deposited on Zenodo (https://doi.org/10.5281/zenodo.7406665).
References
Hand, T. W. & Reboldi, A. Production and function of immunoglobulin A. Annu. Rev. Immunol. 39, 695–718 (2021).
Martinoli, C., Chiavelli, A. & Rescigno, M. Entry route of Salmonella typhimurium directs the type of induced immune response. Immunity 27, 975–984 (2007).
Moor, K. et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature 544, 498–502 (2017).
Fagarasan, S. et al. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science 298, 1424–1427 (2002).
Altmann, S. W. et al. Niemann–Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 303, 1201–1204 (2004).
Cyster, J. G., Dang, E. V., Reboldi, A. & Yi, T. 25-Hydroxycholesterols in innate and adaptive immunity. Nat. Rev. Immunol. 14, 731–743 (2014).
Hannedouche, S. et al. Oxysterols direct immune cell migration via EBI2. Nature 475, 524–527 (2011).
Kelly, L. M., Pereira, J. P., Yi, T., Xu, Y. & Cyster, J. G. EBI2 guides serial movements of activated B cells and ligand activity is detectable in lymphoid and nonlymphoid tissues. J. Immunol. 187, 3026–3032 (2011).
Trindade, B. C. et al. Intracellular sterol sensing controls intestinal B cell differentiation. Immunity 54, 2273–2287 (2021).
Emgård, J. et al. Oxysterol sensing through the receptor GPR183 promotes the lymphoid-tissue-inducing function of innate lymphoid cells and colonic inflammation. Immunity 48, 120–132.e8 (2018).
Russell, D. W. The enzymes,regulation, and genetics of bile acids synthesis. Annu. Rev. Biochem. 72, 137–174 (2003).
Griffiths, W. J., Abdel-Khalik, J., Crick, P. J., Yutuc, E. & Wang, Y. New methods for analysis of oxysterols and related compounds by LC–MS. J. Steroid Biochem. Mol. Biol. 162, 4–26 (2016).
Borah, K. et al. A quantitative LC-MS/MS method for analysis of mitochondrial-specific oxysterol metabolism. Redox Biol. 36, 101595 (2020).
Folch, J., Lees, M. & Sloane Stanley, G. H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–550 (1957).
Baumana, D. R. et al. 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc. Natl Acad. Sci. USA 106, 16764–16769 (2009).
Reboldi, A. et al. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science 345, 679–684 (2014).
Vaishnava, S. et al. The antibacterial lectin RegIIIγ promotes the spatial segregation of microbiota and host in the intestine. Science 334, 255–258 (2011).
Davis, H. R. et al. Niemann–Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 279, 33586–33592 (2004).
Endo, A., Kuroda, M. & Tsujita, Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinum. J. Antibiotics 29, 1346–1348 (1976).
Wang, D. Q.-H. Regulation of intestinal cholesterol absorption. Annu. Rev. Physiol 69, 221–248 (2007).
Repa, J. J. et al. Disruption of the sterol 27-hydroxylase gene in mice results in hepatomegaly and hypertriglyceridemia reversal by cholic acid feeding. J. Biol. Chem. 275, 39685–39692 (2000).
Joseph, S. B., Castrillo, A., Laffitte, B. A., Mangelsdorf, D. J. & Tontonoz, P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 9, 213–219 (2003).
Randolph, G. J. & Miller, N. E. Lymphatic transport of high-density lipoproteins and chylomicrons. J. Clin. Invest. 124, 929–935 (2014).
Tso, P. & Gollamudi, S. R. Pluronic L-81: a potent inhibitor of the transport of intestinal chylomicrons. Am. J. Physiol. 247, G32–G36 (1984).
Johnston, T. P. & Palmers, W. K. Mechanism of Poloxamer 407-induced hyperglyceridemia in the rat. Biochem. Pharmacol. 46, 1037–1042 (1993).
Huang, L.-H. et al. Postprandial chylomicron output and transport through intestinal lymphatics are not impaired in active Crohn’s disease. Gastroenterology 159, 1955–1957.e2 (2020).
Schippers, A. et al. Mucosal addressin cell-adhesion molecule-1 controls plasma-cell migration and function in the small intestine of mice. Gastroenterology 137, 924–933 (2009).
Palm, N. W. et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158, 1000–1010 (2014).
Tellier, J. et al. Blimp-1 controls plasma cell function through the regulation of immunoglobulin secretion and the unfolded protein response. Nat. Immunol. 17, 323–330 (2016).
Jiang, H. et al. Unfolded protein response inducers tunicamycin and dithiothreitol promote myeloma cell differentiation mediated by XBP-1. Clin. Exp. Med. 15, 85–96 (2015).
Lindner, C. et al. Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine. J. Exp. Med. 209, 365–377 (2012).
Zhi, J. et al. Metabolic profiles of minimally absorbed orlistat in obese/overweight volunteers. J. Clin. Pharm. 11, 1006–1011 (1996).
Lopes, N. et al. Distinct metabolic programs established in the thymus control effector functions of γδ T cell subsets in tumor microenvironments. Nat. Immunol. 22, 179–192 (2021).
Gatto, D., Bauer, M., Martin, S. W. & Bachmann, M. F. Heterogeneous antibody repertoire of marginal zone B cells specific for virus-like particles. Microbes Infect. 9, 391–399 (2007).
Rojas, O. L. et al. Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell 176, 610–624.e18 (2019).
Cyster, J. G., Dang, E. V., Reboldi, A. & Yi, T. 25-Hydroxycholesterols in innate and adaptive immunity. Nat. Rev. Immunol. 14, 731–743 (2014).
Guzman, M. et al. An integrin αEβ7-dependent mechanism of IgA transcytosis requires direct plasma cell contact with intestinal epithelium. Mucosal Immunol. 14, 1347–1357 (2021).
Lu, E., Dang, E. V., McDonald, J. G. & Cyster, J. G. Distinct oxysterol requirements for positioning naïve and activated dendritic cells in the spleen. Sci. Immunol. 2, eaal5237 (2017).
Masopust, D. & Soerens, A. G. Tissue-resident T cells and other resident leukocytes. Annu. Rev. Immunol. 37, 1–26 (2019).
Hayward, S. L. et al. Environmental cues regulate epigenetic reprogramming of airway-resident memory CD8+ T cells. Nat. Immunol. 21, 309–320 (2020).
Pan, Y. et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 543, 1–17 (2017).
Penny, H. A. et al. Rhythmicity of intestinal IgA responses confers oscillatory commensal microbiota mutualism. Sci. Immunol. 7, eabk2541 (2022).
Weichhart, T., Hengstschläger, M. & Linke, M. Regulation of innate immune cell function by mTOR. Nat. Rev. Immunol. 15, 599–614 (2015).
Chiang, J. Y. L. & Ferrell, J. M. Bile acids as metabolic regulators and nutrient sensors. Annu. Rev. Nutr. 39, 175–200 (2019).
Wahlström, A., Sayin, S. I., Marschall, H.-U. & Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 24, 41–50 (2016).
Sayin, S. I. et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17, 225–235 (2013).
Pabst, O. & Slack, E. IgA and the intestinal microbiota: the importance of being specific. Mucosal Immunol. 13, 1–10 (2019).
Kotas, M. E. & Locksley, R. M. Why innate lymphoid cells? Immunity 48, 1081–1090 (2018).
Kanneganti, T.-D. & Dixit, V. D. Immunological complications of obesity. Nat. Immunol. 13, 707–712 (2012).
Frascoli, M., Reboldi, A. & Kang, J. Dietary cholesterol metabolite regulation of tissue immune cell development and function. J. Immunol. 209, 645–653 (2022).
Gatto, D. et al. The chemotactic receptor EBI2 regulates the homeostasis, localization and immunological function of splenic dendritic cells. Nat. Immunol. 14, 446–453 (2013).
Pereira, J. P., Kelly, L. M., Xu, Y. & Cyster, J. G. EBI2 mediates B cell segregation between the outer and centre follicle. Nature 460, 1122–1126 (2009).
Chen, S., Lee, B., Lee, A. Y.-F., Modzelewski, A. J. & He, L. Highly efficient mouse genome editing by CRISPR ribonucleoprotein electroporation of zygotes. J. Biol. Chem. 291, 14457–14467 (2016).
Moor, K. et al. Analysis of bacterial-surface-specific antibodies in body fluids using bacterial flow cytometry. Nat. Protoc. 11, 1531–1553 (2016).
Reboldi, A. et al. IgA production requires B cell interaction with subepithelial dendritic cells in Peyeras patches. Science 352, aaf4822 (2016).
Miyoshi, H. & Stappenbeck, T. S. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat. Protoc. 8, 2471–2482 (2013).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Acknowledgements
This work was supported by the Kenneth Rainin Foundation Innovator Award, Charles H. Hood Foundation Child Health Research Awards Program, Leukemia and Lymphoma Society New Idea Award and Multiple Myeloma Research Fellowship and National Institutes of Health (NIH) grant (no. AI155727 to A.R.). S.C. was supported through the American Association of Immunologists Careers in Immunology Fellowship Program and Charles A. King Trust Postdoctoral Research Fellowship Award. This work was also supported by the NIH (grant nos. AI040098 to J.G.C., DK070855 to L.V.H., DP1DK109668 and DP1DK126190 to G.J.R). We acknowledge the University of Massachusetts Chan Flow Cytometry Core Facility for access to sorting services. We thank J. R. Muppidi and E. V. Dang for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
S.C. and A.R. conceived the study, developed the concept, designed the experiments and wrote the paper. S.C. performed the experiments, analyzed the data and interpreted the results. A.B., K.H., Y.L., N.K.H.Y., Y.X., B.M. and S.K.B. performed the experiments. R.B. and J.G.C. provided critical mouse lines. G.J.R. and N.K.H.Y. provided human chylomicron samples. L.V.H. and Y.L. provided epithelial cells from GF mice and Villin-cre:MyD88 floxed mice. All the authors contributed to the review and editing of the manuscript. A.R. reviewed the data and supervised the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Oliver Pabst and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: L. A. Dempsey in collaboration with the Nature Immunology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
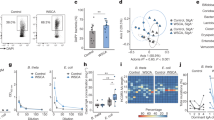
Extended Data Fig. 1 7α,25-HC production is mainly restricted to the duodenum.
a, Ch25h and Cyp7b1 mRNA expression (A.U.) in duodenum and ileum of C57BL/6 mice. b, 7α,25-HC reduction in bile acids precursor (4-cholestern-7 α,25-ol-3-one) by HSD3B7. c, Hsd3b7 mRNA expression (A.U.) in duodenum and ileum of C57BL/6 mice. d, Relative migration of GPR183+ cells with tissue lipid extracts from duodenum and ileum. e, mRNA quantification of Ch25h, Cyp7b1, Hsd3b7 in IECs and LP of C57BL/6 mice. f, Representative flow cytometry plot and number of GPR183+ cells migrating upon exposure to lipid extracts from duodenal and ileal IECs and lamina propria used in Fig. 1c, d. g, Schematic depiction of in vitro 25-HC quantification strategy. The results were pooled from three independent experiments (a,c,d and e)(n = 5 mice per group). Statistics were measured as two-sided unpaired Student’s t-test (*p < 0.05,**p < 0.01,***p < 0.001) in (a,c,d and e) with Bonferroni correction. Exact P values and adjustments are provided in Source data.The error bars represent the mean ± s.e.m.
Extended Data Fig. 2 Inhibition of chylomicron production reduces 7α,25-HC, but not 25-HC, in the lymph.
a, Quantification of GPR183 ligand (Lipid) and precursors (LipidHsd3b7+Cyp7b1) in lymph of mice treated for 24 hours with NF or HCF. b, Representative staining of lipids by Oil Red O and Hematoxylin counterstaining of 7 μm duodenum section from mice treated with 3% of PL81 (vol/vol) or vehicle (saline). Scale bar is 100 μm. c, Plasma of mice untreated (red-clear apparency) or treated (milky apparency) with Poloxamer 407 to prevent chylomicrons clearance. d, M12 migration assay with lipid extracts (GPR183 ligand) from lymph and plasma of mice upon Poloxamer 407 or saline treatment. e, Triglycerides (TG) quantification in chylomicron and non-chylomicron fractions isolated from human plasma at the indicated time after exposure to lipid-based meal. The results were pooled from three independent experiments (a and d)(n = 5 mice per group); (e)(n = 3 biologically independent samples). Statistics were measured as two-sided unpaired Student’s t-test (***p < 0.001,****p < 0.0001) in (a,d) and two-way ANOVA with Bonferroni correction (***p < 0.001, ****p < 0.0001) in (e). Exact P values and adjustments are provided in Source data. The error bars represent the mean ± s.e.m.
Extended Data Fig. 3 25-HC pathway controls duodenum IgA secretion.
a, Quantification of Ch25h mRNA by RT-qPCR in intestinal epithelial cells from VillincreCh25hfl/fl (IECΔCh25h) and littermate controls (IECWt). b, Western blot of CH25H (~31.74KDa), EPCAM (~40KDa) and β-ACTIN (~42KDa) quantification in IECs of IECΔCh25h and IECWt mice. Samples derived from the same experiment and gel were processed in parallel. c, Area and intensity quantification of IgA+ spots from lamina propria of IECWt and IECΔCh25h mice treated with normal food (NF) or 2% of cholesterol food (HCF). Each dot represents single IgA+spot. d, Tamoxifen inducible knockout model of Ch25h in IECs, upon restriction of PCs circulation by i.p. injection of 100 μg of anti-MadCam. e, Quantification of Ch25h mRNA by RT-qPCR in intestinal epithelial cells from IECΔCh25h and littermate controls (IECWt) two days after tamoxifen injection. f, Representative IgA ELISpot and compiled data of duodenum and (g) ileum lamina propria cells from IECWt and IECΔCh25h mice after Tamoxifen treatment. The results were pooled from three independent experiments (a)(n = 5-6 mice per group); (b)(n = 3 independent samples per group); (c)(n = 76–114 cells per group); (e)(n = 5 mice per group); (g)(n = 7 mice per group) and two independent experiments (f)(n = 4 mice per group). Statistics were calculated with two-sided unpaired Student’s t-test (***P < 0.001) in (a,f,g), two-way ANOVA (****P < 0.0001,***P < 0.001,**P < 0.01,*P < 0.05) in (c) and one-way ANOVA with Bonferroni correction (*p < 0.05, ***p < 0.001, ****P < 0.0001) in (e). Exact P values and adjustments are provided in Source data. The error bars represent the mean ± s.e.m.
Extended Data Fig. 4 Inhibition of cholesterol uptake, but not cholesterol biosynthesis, controls duodenum IgA secretion.
a, Representative IgA ELISpot and compiled data of duodenum lamina propria of mice treated with 10 mg/kg (body weight) of EZT or 20 mg/kg (body weight) of Mevastatin or Vehicle (DMSO) for 24 hours. b, Area and intensity quantification of IgA+ spots from lamina propria of IECWt and IECΔCh25h mice treated with EZT or DMSO as in Fig. 2f. Each dot represents single IgA+spot. c, Total number of PCs measured by flow cytometry and gated on B220- IgA+ cells in duodenal lamina propria of IECWt and IECΔCh25h mice treated with NF, HCF or EZT. d, Representative IgA ELISpot of bone marrow of IECWt and IECΔCh25h mice treated with EZT or DMSO. e, Analysis of total microbiome populations in luminal small intestine of IECWt and IECiΔCh25h mice by 16S. The results were pooled from three independent experiments (a)(n = 5 mice per group); (b)(n = 104–117 cells per group); (c)(n = 4–7 mice per group); (d)(n = 6 mice per group); (e)(n = 3 mice per group). Statistics were calculated with two-sided unpaired Student’s t-test (***P < 0.001) in (c,d), two-way ANOVA (****P < 0.0001,***P < 0.001,**P < 0.01,*P < 0.05) in (b) and one-way ANOVA with Bonferroni correction (*p < 0.05, ***p < 0.001, ****P < 0.0001) in (a). Exact P values and adjustments are provided in Source data. The error bars represent the mean ± s.e.m.
Extended Data Fig. 5 Lack of Ch25h in IECs and cholesterol uptake inhibition control CD98 expression IgA secreting plasma cells in the duodenum.
a, Number of CD98hi and CD98lo PCs and total IgA+PC in lamina propria of mice treated with 10 mg/kg (body weight) of EZT or vehicle and euthanized 24 hours later. b, Representative flow cytometry and frequency and total number of IgA+ PCs in the ileum of IECWt and IECiΔCh25h mice (c). d, Representative flow cytometry and compiled quantification of frequency and total number of IgA+ PCs in the ileum of mice treated for 24 hours with NF, HCF and EZT (e). f, Representative flow cytometry and frequency of IgA+PCs in duodenum lamina propria of mice treated with EZT or Mevastatin or vehicle and eutha- nized 24 hours later. g, Flow cytometry and frequency of IgA+ PCs in BM of mice treated with EZT and Mevastatin as in (f). The results are pooled from three independent experiments (a)(n = 5 mice per group); (b and c)(n = 7 mice per group) and two indepen- dent experiments in (d,e,f and g)(n = 4 mice per group). Statistics were measured as two-sided unpaired Student’s t-test (**p < 0.01,***p < 0.001) in (a,b and c) and one-way ANOVA with Bonferroni correction (***p < 0.001) in (d,e,f and g). Exact P values and adjustments are provided in Source data.The error bars represent the mean ± s.e.m.
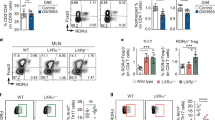
Extended Data Fig. 6 Ch25h-expressing epithelial cells restrain BLIMP1 upregulation and antigen-specific mucosal response in intestinal plasma cells.
a, IgA ELISA of intestinal lavages from wild type mice treated for two consecutive days with BTZ and injected with GFP+ cells (1 × 106). Intestinal lavages were collected at the indicated days after BTZ injection. b, Experimental model of BTZ treatment and adoptive transfer of 1 × 106 cells from lamina propria of IgMa mice treated 10 days with antibiotics (AVNM) or PBS into IgMb mice. c, Representative IgAb ELISpot of LP PCs and CD98hiIgA+ PCs frequency from mice treated as (b). d, Experimental model of BTZ treatment and adoptive transfer of 1 × 106 cells from lamina propria of Prdm1YFP+ reporter mouse into IECWt and I IECCh25h mice. e, Representative flow cytometry plot and compiled quantification of BLIMP1+ PCs in bone marrow of IECWt and IECΔCh25h mice treated as in (d). f, Area and intensity quantification of IgA+ spots from lamina propria of IECWt and IECCh25h mice treated with BTZ and injected with lamina propria cells from mice infected with non-replicative ΔAroA Salmonella Typhimurium. Each dot represents single IgA+spot. g, Salmonella-specific IgA ELISpot in bone marrow and spleen of mice treated as in Fig. 4j. The results are pooled from two independent experiments (a)(n = 2 mice per group) and three independent experiments (c,e and g)(n = 4–6 mice per group). Statistics were measured as two-sided unpaired Student’s t-test (**p < 0.01; ***p < 0.001; ****p < 0.0001) in (a,c,e,f and g). Exact P values and adjustments are provided in Source data.The error bars represent the mean ± s.e.m.
Extended Data Fig. 7 Sensing of GPR183 ligand in plasma cells depends on intestinal lipoprotein lipase activity.
a, Representative flow cytometry plot and compile frequency of secreting IgA+ PCs (CD98hiIgA+) in mice treated for 24 hours with a lipoprotein lipases (LPL) inhibitor, Orlistat, or DMSO. b, GPR183 ligand quantification in lamina propria, lymph and plasma of mice treated for two consecutive days with 50 mg/kg (body weight) of Orlistat. c, Quantification of LPL activity by fluorometric assay in duodenum lamina propria of mice treated as in (a and b). d, Illustration of the mechanism regulating 7α,25-HC trafficking from IECs to lamina propria. IEC-produced chylomicrons enter the intestinal lacteal and reach the lamina propria by LPL-mediated degradation or travel versus lymphoid organs. e, f, Quantification of PC distance from lymphatics in the indicated mice used in Fig. 1c, d. Each dot represents a single PC. g, Representative immunofluorescence of IgA+PCs, lymphatics and DAPI in ileum sections from wild type and IECΔCh25h mice used in Fig. 6a, b, d. Scale bar is 100 μm. h, Quantification of PC frequency at the indicated distance from lymphatics. Each dot represents the average of PCs distance measured in sections from n = 4 mice per each treatment. i, Quantifi- cation of PCs distance from lymphatics in section of ileum from wild type and IECΔCh25h mice. Each dot represents a single PC. j, Frequency of α chain CD103 (αE) in CD98lo and CD98hi cells from duodenum of IECWt and IECΔCh25h mice used in Fig. 6a, b, d. The results were pooled from three independent experiments (a and b)(n = 6–7 mice per group); (e and f)(n = 136–210 cells); (h and i)(n = 4 mice per group and n = 90–97 cells (i)) and two independent experiments (c and i)(n = 3–4 mice per group). Statistics were calculated with two-sided unpaired Student’s t-test (**P < 0.01,***p < 0.001,****P < 0.0001) in (a,b,c,e,f, h and i) and two-way ANOVA with Bonferroni correction (*p < 0.05, ***p < 0.001, ****p < 0.0001) in (j). Exact P values are provided in Source data. The error bars represent the mean ± s.e.m.
Extended Data Fig. 8 Diet-derived oxysterols shape spatial localization of IgA+ plasma cells in lamina propria.
a, Representative immunofluorescence of duodenum sections from mice treated for 24 hrs with NF, HCF and VF. b, Quantification of PC frequency at the indicated distance from lymphatics (LYVE1+) in duodenum of mice treated as in (a). Each dot represents the average of PCs distance measured from n = 6 mice per each treatment. c, Quantification of PCs distance from lymphatics in mice treated with the indicated food changes. Each dot represents a single PC. d, Representative IgA ELISpot and compiled data from duodenum of untreated or fasted mice for 12 hours. e, Frequency of IgA+CD98hi cells analyzed by flow cytometry in LP of mice fasted for 12hrs, or untreated. The results were pooled from three experiments (a,b and c)(n = 7 mice per group and n = 50–145 cells (c)) and two independent experiments (d, e)(n = 3–5 mice per group). Statistics were calculated with two-way ANOVA with Bonferroni correction (*p < 0.05, **p < 0.01,***p < 0.001, ****p < 0.0001) in (b and c) and two-sided unpaired Student’s t-test (**P < 0.01,****P < 0.0001) in (d and e). Exact P values are provided in Source data. The error bars represent the mean ± s.e.m.
Extended Data Fig. 9 Modulation of intestinal oxysterol concentration shapes intestinal plasma cell positioning.
a, Quantification of PC distance from lymphatics in mice treated with the indicated food changes. Each dot represents a single PC. b, M12 migration assay with lipid extracts from IECs of mice fasted for 12hrs, refed once with HCF and harvested after 3 or 12 hrs. c, Representative immunofluorescence and frequency of IgA+ PCs at certain distance to lymphatics in duodenum of mice treated with 3% of Pluronic81 (PL81)(vol/vol), 10 mg/kg (body weight) of Ezetimibe (Ezt), 50 mg/kg (body weight) of Orlistat or vehicle (saline or DMSO). Each dot represents the average of PCs distance measured in a total of n = 7 mice per each treatment or (d) single PCs. Scale bar is 50 μm. The results are pooled from three independent experiments (a)(n = 49–85 cells per group); (b)(n = 7 mice per group); (c and d)(n = 7–11 mice per group). Statistics were measured by two-way ANOVA Bonferroni correction (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001) in (a and b) or two-sided unpaired Student’s t-test (**p < 0.01, ***p < 0.001) in (c and d). Exact P values and adjustments are provided in Source data. The error bars represent the mean ± s.e.m.
Extended Data Fig. 10 GPR183 pharmacological inhibition shapes plasma cell dynamics and antigen-specific response.
a, Relative migration of lamina propria PCs from NIBR189 or vehicle (DMSO) treated mice and with the indicated concentration of GPR183L. b, Representative immunofluorescence of duodenum sections from mice treated twice with GPR183 inhibitor, NIBR189, or vehicle (DMSO). Scale bar is 50 μm. c, Quantification of IgA+PCs frequency at certain distance from lymphatics (LYVE1+) showed as violin plot. Each dot represents the average of PCs frequency at the indicated range of n = 7 mice per each treatment. d, Quantification of PCs distance from lymphatics upon GPR183 inhibition. Each dot represents a single PC. The results were pooled from two independent experiments (a)(n = 3 mice pre group) and three independent experiments (b,c and d) (n = 7 mice per group or n = 62 cells (d)). Statistics were measured as two-sided unpaired Student’s t-test (***p < 0.001,****p < 0.0001) in (a,c and d). Exact P values and adjustments are provided in Source data. The error bars represent the mean ± s.e.m.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5 and Tables 1–3.
Supplementary Video 1
A 3D representation of PC localization in IECWt villi.
Supplementary Video 2
A 3D representation of PC localization in IECΔCh25h villi.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed immunoblot.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ceglia, S., Berthelette, A., Howley, K. et al. An epithelial cell-derived metabolite tunes immunoglobulin A secretion by gut-resident plasma cells. Nat Immunol 24, 531–544 (2023). https://doi.org/10.1038/s41590-022-01413-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-022-01413-w