Abstract
Despite tumor-associated macrophages (TAMs) playing a key role in shaping the tumor microenvironment (TME), the mechanisms by which TAMs influence the TME and contribute to cancer progression remain unclear. Here, we show that the N6-methyladenosine reader YTHDF2 regulates the antitumor functions of TAMs. YTHDF2 deficiency in TAMs suppressed tumor growth by reprogramming TAMs toward an antitumoral phenotype and increasing their antigen cross-presentation ability, which in turn enhanced CD8+ T cell-mediated antitumor immunity. YTHDF2 deficiency facilitated the reprogramming of TAMs by targeting interferon-γ−STAT1 signaling. The expression of YTHDF2 in TAMs was regulated by interleukin-10−STAT3 signaling. Selectively targeting YTHDF2 in TAMs using a Toll-like receptor 9 agonist-conjugated small interfering RNA reprogrammed TAMs toward an antitumoral phenotype, restrained tumor growth and enhanced the efficacy of PD-L1 antibody therapy. Collectively, our findings describe the role of YTHDF2 in orchestrating TAMs and suggest that YTHDF2 inhibition is an effective approach to enhance cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The scRNA-seq, m6A-seq and RIP–seq data reported in this paper are deposited in the Gene Expression Omnibus under accession numbers GSE188400 and GSE189193. The remaining data that support our study’s findings are available from the corresponding authors upon request. Source data are provided with this paper.
Code availability
Publicly available packages were used to perform the analysis of scRNA-seq data. Detailed R scripts for figures are available at https://github.com/ShoubaoMa/scRNA-seq_Ythdf2_Macrophage.
References
Murciano-Goroff, Y. R., Warner, A. B. & Wolchok, J. D. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 30, 507–519 (2020).
Schupp, J. et al. Targeting myeloid cells in the tumor sustaining microenvironment. Cell Immunol. 343, 103713 (2019).
Engblom, C., Pfirschke, C. & Pittet, M. J. The role of myeloid cells in cancer therapies. Nat. Rev. Cancer 16, 447–462 (2016).
Ruffell, B. et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 26, 623–637 (2014).
Qian, B. Z. & Pollard, J. W. Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51 (2010).
Broz, M. L. et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26, 638–652 (2014).
Li, X., Ma, S., Deng, Y., Yi, P. & Yu, J. Targeting the RNA m6A modification for cancer immunotherapy. Mol. Cancer 21, 76 (2022).
Wang, L. et al. m6A RNA methyltransferases METTL3/14 regulate immune responses to anti-PD-1 therapy. EMBO J. 39, e104514 (2020).
Yang, S. et al. m6A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nat. Commun. 10, 2782 (2019).
Liu, Y. et al. Tumors exploit FTO-mediated regulation of glycolytic metabolism to evade immune surveillance. Cell Metab. 33, 1221–1233 (2021).
Li, N. et al. ALKBH5 regulates anti-PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment. Proc. Natl Acad. Sci. USA 117, 20159–20170 (2020).
Su, R. et al. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell 38, 79–96 (2020).
Yin, H. et al. RNA m6A methylation orchestrates cancer growth and metastasis via macrophage reprogramming. Nat. Commun. 12, 1394 (2021).
Han, D. et al. Anti-tumour immunity controlled through mRNA m6A methylation and YTHDF1 in dendritic cells. Nature 566, 270–274 (2019).
Dong, L. et al. The loss of RNA N6-adenosine methyltransferase Mettl14 in tumor-associated macrophages promotes CD8+ T cell dysfunction and tumor growth. Cancer Cell 39, 945–957 (2021).
Xiong, J. et al. Lactylation-driven METTL3-mediated RNA m6A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol. Cell. 82, 1660–1677 (2022).
Wang, X. et al. N6-methyladenosine modulates messenger RNA translation efficiency. Cell 161, 1388–1399 (2015).
Wang, X. et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120 (2014).
Li, A. et al. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. 27, 444–447 (2017).
Shi, H. et al. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 27, 315–328 (2017).
Dixit, D. et al. The RNA m6A reader YTHDF2 maintains oncogene expression and is a targetable dependency in glioblastoma stem cells. Cancer Discov. 11, 480–499 (2021).
Einstein, J. M. et al. Inhibition of YTHDF2 triggers proteotoxic cell death in MYC-driven breast cancer. Mol. Cell 81, 3048–3064 (2021).
Yoshihara, K. et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 4, 2612 (2013).
Darmanis, S. et al. Single-cell RNA-seq analysis of infiltrating neoplastic cells at the migrating front of human glioblastoma. Cell Rep. 21, 1399–1410 (2017).
Zhang, L. et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell 181, 442–459 (2020).
Azizi, E. et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell 174, 1293–1308 (2018).
Clausen, B. E., Burkhardt, C., Reith, W., Renkawitz, R. & Forster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 (1999).
Gholamin, S. et al. Disrupting the CD47-SIRPα anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci. Transl. Med. 9, eaaf2968 (2017).
Muntjewerff, E. M., Meesters, L. D. & van den Bogaart, G. Antigen cross-presentation by macrophages. Front. Immunol. 11, 1276 (2020).
Dixon, K. O. et al. TIM-3 restrains anti-tumour immunity by regulating inflammasome activation. Nature 595, 101–106 (2021).
Efremova, M., Vento-Tormo, M., Teichmann, S. A. & Vento-Tormo, R. CellPhoneDB: inferring cell-cell communication from combined expression of multi-subunit ligand–receptor complexes. Nat. Protoc. 15, 1484–1506 (2020).
Le Bon, A. et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4, 1009–1015 (2003).
Lawrence, T. & Natoli, G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 11, 750–761 (2011).
Mapperley, C. et al. The mRNA m6A reader YTHDF2 suppresses proinflammatory pathways and sustains hematopoietic stem cell function. J. Exp. Med. 218, e20200829 (2021).
Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Noy, R. & Pollard, J. W. Tumor-associated macrophages: from mechanisms to therapy. Immunity 41, 49–61 (2014).
Murray, P. J. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr. Opin. Pharmacol. 6, 379–386 (2006).
Roberts, T. C., Langer, R. & Wood, M. J. A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 19, 673–694 (2020).
Krieg, A. M. Development of TLR9 agonists for cancer therapy. J. Clin. Invest. 117, 1184–1194 (2007).
Kortylewski, M. et al. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat. Biotechnol. 27, 925–932 (2009).
Maurer, T. et al. CpG–DNA aided cross-presentation of soluble antigens by dendritic cells. Eur. J. Immunol. 32, 2356–2364 (2002).
Edelson, B. T. et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. J. Exp. Med. 207, 823–836 (2010).
Xu, J. et al. Single-cell profiling reveals transcriptional signatures and cell–cell crosstalk in anti-PLA2R positive idiopathic membranous nephropathy patients. Front. Immunol. 12, 683330 (2021).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Moon, E. K. et al. Blockade of programmed death 1 augments the ability of human T cells engineered to target NY-ESO-1 to control tumor growth after adoptive transfer. Clin. Cancer Res. 22, 436–447 (2016).
Robbins, P. F. et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J. Immunol. 180, 6116–6131 (2008).
Abram, C. L., Roberge, G. L., Hu, Y. & Lowell, C. A. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J. Immunol. Methods 408, 89–100 (2014).
Yankova, E. et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature 593, 597–601 (2021).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Powles, T. et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515, 558–562 (2014).
Ma, S. et al. The RNA m6A reader YTHDF2 controls NK cell antitumor and antiviral immunity. J. Exp. Med. 218, e20210279 (2021).
Wang, F. et al. Crosstalks between mTORC1 and mTORC2 variagate cytokine signaling to control NK maturation and effector function. Nat. Commun. 9, 4874 (2018).
Wang, Y. et al. The IL-15–AKT–XBP1s signaling pathway contributes to effector functions and survival in human NK cells. Nat. Immunol. 20, 10–17 (2019).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Kramer, A., Green, J., Pollard, J. Jr. & Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30, 523–530 (2014).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Zhang, Y. et al. Model-based analysis of ChIP–seq (MACS). Genome Biol. 9, R137 (2008).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Freund, E. C. et al. Efficient gene knockout in primary human and murine myeloid cells by non-viral delivery of CRISPR–Cas9. J. Exp. Med. 217, e20191692 (2020).
Singhal, S. et al. Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Sci. Transl. Med. 11, eaat1500 (2019).
Tang, Z., Kang, B., Li, C., Chen, T. & Zhang, Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 47, W556–W560 (2019).
He, S. et al. MicroRNAs activate natural killer cells through Toll-like receptor signaling. Blood 121, 4663–4671 (2013).
Acknowledgements
We thank the City of Hope Center for Comparative Medicine, Analytical Cytometry Core, Pathology Core, Integrative Genomics Core and DNA/RNA Synthesis Laboratory for performing some work. We thank F. Chiuppesi and V. Karpinski for analyzing the ELISpot data. The following reagents were obtained through the NIH Tetramer Core Facility: H-2Kb SIINFEKL-PE tetramers (contract number 75N93020D00005). We thank A. Li, Z. Yin and A. Misra for IHC staining and reviewing. We thank H. Yu for providing Stat3f/fLyz2Cre mice. We thank S. Albelda for providing the NY-ESO-1-reactive Ly95 TCR plasmid. This work was supported by grants from the National Institutes of Health (CA210087, CA265095 and CA163205 to M.C.; NS106170, AI129582, CA247550, CA264512, CA266457 and CA223400 to J.Y.), the Leukemia and Lymphoma Society (1364-19 to J.Y.) and the 2021 Exceptional Project Award from the Breast Cancer Alliance (to J.Y.). Images were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
S.M., J.Y. and M.A.C. conceived and designed the project. S.M. and J.H. designed and conducted the laboratory experiments. S.M., B.S., S.D., T.B. and J.Z. analyzed the data. C.H. and J.C. contributed to the Ythdf2f/f mice. M.K. contributed to the Batf3−/− mice and helped with the oligonucleotide. M.B.B. contributed to the colon cancer biopsies. S.M., M.K., C.H., J.C., M.A.C. and J.Y. wrote, reviewed and/or revised the paper. J.Y. and M.A.C. acquired funding. All authors discussed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Yang-Xin Fu, Li Wu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ioana Visan, in collaboration with the Nature Immunology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 TCGA database and public single-cell RNA-seq data analysis to determine expression pattern of YTHDF2 in cancers and tumor-infiltrating myeloid cells.
a, Boxplots showing the YTHDF2 expression in multiple types of cancers and corresponding normal tissues from RNA-seq results of the TCGA database. Red and blue boxes indicate the tumor group and normal group, respectively. The horizontal line indicates the median value; the box represents the first and third quartiles. Data were analyzed by two-sided unpaired wilcoxon test. b, Scatter plots showing an inverse Spearman’s correlation betweenYTHDF2 expression and immune scores calculated by the ESTIMATE algorithm from the TCGA database. Data are shown as a fitted line with 95% confidence level and were analyzed by linear model. c, The bubble plots showing the positive correlation of YTHDF2 expression with immune-related markers in each type of cancer. d-f, YTHDF2 expression in tumor-infiltrating myeloid cells and corresponding normal tissues from single-cell RNA-seq datasets of GBM (d), COAD (e), and BRCA (f). P values were calculated by two-sided unpaired wilcoxon test. Abbreviation for TCGA cancer types: BRCA, Breast invasive carcinoma; CHOL, Cholangiocarcinoma; COAD, Colon adenocarcinoma; DLBC, Lymphoid neoplasm diffuse large B-cell lymphoma; GBM, Glioblastoma multiforme; LGG, Brain lower grade glioma; PAAD, Pancreatic adenocarcinoma; READ, Rectum adenocarcinoma; SKCM, Skin cutaneous melanoma; STAD, Stomach adenocarcinoma; TGCT, Testicular germ cell tumors; THYM, Thymoma; UCEC, Uterine corpus endometrial carcinoma; UCS, Uterine carcinosarcoma.
Extended Data Fig. 2 YTHDF2 deficiency in myeloid cells does not affect the percentages, absolute numbers, or functions of tumor-infiltrating MDSCs and DCs.
a, b, qPCR (a) (n = 4) and immunoblotting (b) showing the expression of YTHDF2 in splenicCD3−CD19−CD49b−CD11b+ myeloid cells from Ythdf2f/f and Ythdf2cKO mice. c, d, qPCR (c) (n = 4) and immunoblotting (d) showing the expression of YTHDF2 in bone marrow-derived macrophages (BMDMs) from Ythdf2f/f and Ythdf2cKO mice. e-g, Gating strategy (e), percentages (f), and absolute numbers (g) of CD11c+MHC-II+ DCs, CD11b+F4/80+ macrophages, CD11b+Ly6c+ monocytes, and CD11b+Ly6G+ neutrophils in the spleen of Ythdf2f/f and Ythdf2cKO (n = 3). h-k, Percentages (h, j) and absolute numbers (i, k) of tumor-infiltrating CD11b+Gr-1+ MDSCs and CD11b+CD11c+MHC-II+ DCs from Ythdf2f/f and Ythdf2cKO mice on day 14 post-B16-OVA (h, i) or MC38 (j, k) tumor inoculation (n = 5). l, Percentages and representative plots of IFN-γ-producing OT-I CD8+ T cells co-cultured with purified tumor-infiltrated CD11b+Gr-1+ MDSCs from B16-OVA bearing Ythdf2f/f and Ythdf2cKO mice in the presence of SIINFEKEL (n = 3). m, Percentages and representative plots of IFN-γ-producing OT-I CD8+ T cells co-cultured with purified tumor-infiltrated CD11b+CD11c+MHC-II+ DCs from B16-OVA-bearing Ythdf2f/f and Ythdf2cKO mice in the presence of SIINFEKEL (n = 3). n, o, Percentages of IFN-γ-producing OT-I CD8+ T cells co-cultured with purified tumor-infiltrated MDSCs (n) or DCs (o) from B16-OVA-bearing Ythdf2f/f and Ythdf2cKO mice in the presence of ovalbumin protein (n = 3). p, q, Immunoblotting showing the expression of YTHDF2 in BMDCs (p) or splenic DCs (q) from Ythdf2f/f and Ythdf2cKO mice. r, Percentages of tumor-infiltrating CD11b+F4/80+ macrophages after injection with clodronate liposomes or PBS liposomes in B16-OVA-bearing Ythdf2f/f and Ythdf2cKO mice (Ythdf2f/f + PBS liposome, n = 6; Ythdf2cKO + PBS liposome, n = 5; Ythdf2cKO + clodronate liposome, n = 4). s, Schematic diagram showing s.c. co-injection of B16-OVA cells with BMDMs from CD45.2 mice into CD45.1 mice and representative plots of the macrophages in the tumor tissues on day 14 post-B16-OVA tumor inoculation. Data are shown as mean ± SD and were analyzed with an unpaired two-tailed t-test (a-o) or one-way ANOVA with the Holm-Šídák post-test (r). Data in a, b, c, d, f-r are representative of at least two independent experiments. NS, not significant.
Extended Data Fig. 3 YTHDF2 deficiency in myeloid cells does not affect phagocytosis by macrophages and CD4+ T and NK cells in the tumor microenvironment.
a, b, Median fluorescence intensity (MFI, a) and representative histogram (b) of pHrodo red zymosan bioparticles uptake by anti-tumoral BMDMs from Ythdf2f/f and Ythdf2cKO mice (n = 3). c, B16-OVA tumor growth on day 18 post-B16-OVA tumor inoculation in Rag1−/− mice s.c. transplanted with BMDMs from Ythdf2f/f and Ythdf2cKO mice (n = 3). d, B16-OVA tumor growth on day 13 post-B16-OVA tumor inoculation in Rag1−/− mice that were i.v. injected with CD3+ T cells and s.c. transplanted with BMDMs from Ythdf2f/f and Ythdf2cKO mice (n = 5). e-h, Percentages (e, g) and absolute numbers (f, h) of tumor-infiltrating CD4+ T cells, CD8+ T cells, and CD3−NK1.1+ NK cells from Ythdf2f/f and Ythdf2cKO mice on day 14 post-B16-OVA tumor cell implantation (e, f) or MC38 (g, h) tumor inoculation (n = 5). i, j, Percentages and representative plots of tumor-infiltrating CD4+ IFN-γ+ TH1 cells, CD4+ IL-17A+ TH17 cells, CD4+ Foxp3+ Treg cells, and granzyme B-producing NK cells from Ythdf2f/f and Ythdf2cKO mice on day 14 post B16-OVA (i) or MC38 (j) tumor inoculation (n = 4). Data are shown as mean ± SD and were analyzed with an unpaired two-tailed t-test (a, e-j) or two-way ANOVA with mixed-effects model and adjusted by the Holm-Šídák post-test (c, d). Data in a-j are representative of at least two independent experiments. NS, not significant.
Extended Data Fig. 4 scRNA-seq of CD45+ tumor-infiltrating immune cells.
a, A t-distributed stochastic neighbor embedding (t-SNE) plot from scRNA-seq showing 15 cell clusters of CD45+ tumor-infiltrating immune cells that were sorted from B16-OVA tumor-bearing Ythdf2f/f and Ythdf2cKO mice on day 14 post B16-OVA tumor inoculation. b, Immunoblotting showing expression of iNOS in anti-tumoral BMDMs and expression of Arg1 in pro-tumoral BMDMs from Ythdf2f/f and Ythdf2cKO mice. The numbers below a lane indicate expression of target proteins normalized to expression of β-actin. Data in b are representative of at least two independent experiments.
Extended Data Fig. 5 YTHDF2 deficiency improves macrophage anti-tumoral polarization by targeting IFN-γ-STAT1 signaling.
a, Ingenuity pathway analysis showing the top four pathways enriched in Ythdf2cKO anti-tumoral macrophages compared to Ythdf2f/f anti-tumoral macrophages. P values were determined by hypergeometric test and adjusted for multiple testing using the Benjamini–Hochberg method. b, qPCR showing the expression of IFN-γ response genes in BMDMs from Ythdf2f/f and Ythdf2cKO mice that were treated with IFN-γ (100 ng/ml) for 6 h (n = 3). c, qPCR showing the expression of Stat1 in CD11b+F4/80+ TAMs isolated from tumor tissues of B16-OVA tumor-bearing Ythdf2f/f and Ythdf2cKO on day 14 post B16-OVA tumor inoculation (n = 4). d, Immunoblotting showing the expression of phosphor (p)-STAT1 and STAT1 in CD11b+F4/80+ TAMs isolated from tumor tissues of B16-OVA tumor-bearing Ythdf2f/f and Ythdf2cKO mice on day 14 post-B16-OVA tumor inoculation. e, Immunoblotting showing the expression of STAT1 in BMDMs transfected with Stat1 siRNA or control siRNA. f, Immunoblotting showing the expression of STAT1 in BMDMs nucleofected with Stat1 gRNA or control gRNA. g, Representative histogram of the expression of IFNGR1 in BMDMs nucleofected with Ifngr1 gRNA or control gRNA. h, B16-OVA tumor growth on day 18 post-tumor inoculation in Rag1−/− mice s.c. implanted with BMDMs from Ythdf2f/f and Ythdf2cKO mice together with i.v. injection of CD8+ T cells from IFN-γ−/− mice (n = 4). Data are shown as mean ± SD and were analyzed with an unpaired two-tailed t-test (b, c) or two-way ANOVA with mixed-effects model and adjusted by the Holm-Šídák post-test (h). The numbers below a lane indicate relative expression levels of target proteins normalized to the expression of β-actin. Data in b-h are representative of at least two independent experiments. NS, not significant.
Extended Data Fig. 6 Transcriptome-wide identification of YTHDF2-binding targets in macrophages using m6A-RIP and YTHDF2 RIP.
a, HOMER motif discovery tool showing the m6A motif in BMDMs from both Ythdf2f/f and Ythdf2cKO mice. b, Density distribution of m6A peaks across the length of mRNA. c, Pie chart depicting the fraction of m6A peaks in four transcript segments. d, Volcano plot showing the differentially expressed genes in anti-tumoral macrophages from Ythdf2cKO mice compared with those from Ythdf2f/f mice from RNA-seq. DEGs with absolute log-transformed fold change > 0.25 and adjusted P < 0.05 (determined by two-sided Wilcoxon rank sum test and adjusted using Bonferroni correction) were defined as significant. e, GSEA results showing the top six pathways enriched in Ythdf2cKO anti-tumoral macrophages compared to Ythdf2f/f anti-tumoral macrophages. The P values were calculated by a permutation test. f, GSEA results showing enrichment of IFN-γ response sets in Ythdf2cKO anti-tumoral macrophages compared to Ythdf2f/f anti-tumoral macrophages. g, Heatmaps showing the relative expression of representative genes in Ifng, Tnf, Ifna, Irf7, Tlr4, and Stat1 signaling pathways in BMDMs from Ythdf2f/f and Ythdf2cKO mice from RNA-seq data (n = 2). Transcript methylation is depicted by filled (m6A-modified) or unfilled (non-m6A-modified) circles. h, Scatterplot showing the overlap of high confidence YTHDF2-binding peaks between replicate 1 and replicate 2 in YTHDF2 RIP-seq. i, Density distribution of YTHDF2-binding peaks across the length of mRNA. j, Pie chart depicting the fraction of YTHDF2-binding peaks in four transcript segments.
Extended Data Fig. 7 YTHDF2 is positively regulated by IL-10−STAT3 signaling in TAMs.
a, Immunoblotting showing the expression of YTHDF2 in CD11b+F4/80+ macrophages isolated from spleen or tumor tissues of B16-OVA tumor-bearing C57BL/6 mice on day 14-post B16-OVA tumor inoculation. b, Immunoblotting showing the expression of YTHDF2 in BMDMs stimulated without or with IL-4, TGF-β, IL-10, or IFN-γ for 24 h. c, Immunoblotting showing the expression of YTHDF2 and phospho-Stat3 in BMDMs stimulated with different doses of IL-10 for 24 h. d, Binding sites for STAT3 in the promoter regions of Ythdf2 (predicted from http://jaspar.genereg.net). e, Luciferase reporter assay showing the luciferase activity of Ythdf2 in BMDMs transfected with STAT3 expressing plasmid or empty vector (n = 3). f, ChIP-qPCR showing the binding of STAT3 to the Ythdf2 promoter in IL-10 (10 ng/ml)–treated BMDMs (n = 3). g, Immunoblotting showing the expression of YTHDF2 and phospho-Stat3 in BMDMs from Stat3f/fand Stat3cKO mice stimulated without or with IL-10 (10 ng/ml). Data are shown as mean ± SD and were analyzed with an unpaired two-tailed t-test (e, f). The numbers below a lane indicate the expression of YTHDF2 normalized to the expression of β-actin. Data in a, b, c, e, f, and g are representative of at least two independent experiments. NS, not significant.
Extended Data Fig. 8 TLR9 expression and CpG-Ythdf2 siRNA design as well as its uptake by macrophages associated with anti-tumor effect in vivo.
a, Violin plot showing the Tlr9 expression across 15 cell clusters from scRNA-seq data in this study. b, Sequence of the CpG-linked mouse Ythdf2 siRNA conjugate (CpG–siRNAYthdf2). c, d, Representative histograms showing the expression of Cy3-CpG-siRNAYthdf2 in wild-type BMDMs incubated with various doses of Cy3-CpG-siRNAYthdf2 (c) or with 100 nM Cy3-labeled CpG-Ythdf2 siRNA for the indicated times (d). e, Representative histograms showing the uptake of Cy3-CpG-siRNAYthdf2 by CD11b+F4/80+ TAMs isolated from tumor tissues of B16-OVA-bearing wild-type mice that received intratumoral (i.t.) injection of CpG-siRNAYthdf2. f, qPCR showing the mRNA expression of mRNA Ythdf2 in CD11b+F4/80+ TAMs sorted from tumor tissues of B16-OVA-bearing mice i.t. injected with CpG-siRNAYthdf2 or CpG-siRNACtrl (n = 3). g, h, Representative dot plots and percentages of CD11b+F4/80+iNOS+ anti-tumoral macrophages (g) or h, CD11b+F4/80+Arg1+ pro-tumoral macrophages (h) in tumor tissues from B16-OVA-bearing mice that received i.t. injection of CpG-siRNAYthdf2 or CpG-siRNACtrl (n = 5). i, qPCR showing the mRNA expression of Ythdf2 in CD11b+F4/80+ TAMs isolated from lung tissues of B16-F10-bearing mice that received i.v. injected of CpG-siRNAYthdf2 or CpG-siRNACtrl (n = 3). j, k, Metastatic nodules (j) and percentages of tumor-infiltrating IFN-γ producing CD8+ T cells (k) in the lung of B16F10-bearing mice that received i.v. injection of CpG-siRNAYthdf2, CpG-siRNACtrl, CpG, or PBS (n = 5). l, B16-OVA tumor growth on day 13 post-tumor inoculation in Batf3−/− mice treated with CpG-siRNAYthdf2 or CpG-siRNACtrl (n = 5). m, n, Violin plots showing the TLR9 expression across cell clusters in scRNA-seq datasets of patients with glioblastoma (m) or kidney cancer (n). o, p, Representative histograms showing expression levels of PD-L1 in B16-OVA cells (o) or BMDMs (p) after treatment with IFN-γ for 24 h. Median fluorescence intensity (MFI) is shown. Data are shown as mean ± SD and were analyzed with an unpaired two-tailed t-test (f-i) or one-way ANOVA with the Holm-Šídák post-test (j, k) or two-way ANOVA with the mixed-effects model and adjusted by Holm-Šídák post-test (l). Data in c-l, o, and p are representative of at least two independent experiments.
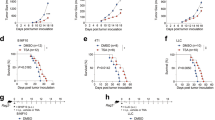
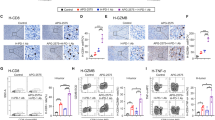
Extended Data Fig. 9 The relationship between YTHDF2 expression in TAMs and overall survival in patients with skin cutaneous melanoma.
a, qPCR showing the mRNA expression of YTHDF2, IL1B, IL6, and IL12 in human MDMs transfected with YTHDF2 siRNA (YTHDF2−/− MDMs) or control siRNA (WT MDMs). Data are shown as mean ± SD and were analyzed by unpaired two-tailed t-test. b, Immunohistochemical staining of YTHDF2 (yellow) plus CD68 (purple) or CD8 (brown) in tumor tissues from patients with lung cancer. A representative specimen of YTHDF2+CD68+ (double positive) with a high (top panel) or low (bottom panel) number of cells is shown (f). Scale bars = 100 μm. Black arrows mark YTHDF2+CD68+ cells. c, Correlations between YTHDF2+CD68+ cells and CD8+ T cells. Data are analyzed by Spearman correlation (n = 32). d, Kaplan–Meier analysis showing the correlation of the expression of YTHDF2 in tumor infiltrated CD68+ macrophages with overall survival in patients with skin cutaneous melanoma (SKCM) from TCGA dataset. P values were calculated using the log-rank test. e, Heatmap of the ordered, z-transformed expression values for YTHDF2 target genes in patients with SKCM from TCGA dataset. f, Correlation of a YTHDF2 target gene signature with overall survival of patients with SKCM comparing high and low quartiles. P values were calculated using the log-rank test. g, Kaplan–Meier analysis showing the correlation of each of the 12 identified YTHDF2 target genes with overall survival of patients with SKCM comparing high and low expression with a 50% cutoff. P values were calculated using the log-rank test. Data in a are representative of at least two independent experiments.
Extended Data Fig. 10 A working model for how reduction in YTHDF2 reprograms tumor-associated macrophages and elicits potent anti-tumor immunity.
TAMs traffic into the TME and respond to IL-10, which upregulates YTHDF2 by IL-10−STAT3 signaling. Ablation of YTHDF2 in TAMs or knock-down of YTHDF2 by CpG-siRNAYthdf2 enhances the stability of STAT1, reprograms TAMs to induce anti-tumoral polarization, thereby facilitating CD8+ T cell anti-tumor response, and eventually suppressing tumor growth.
Supplementary information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, S., Sun, B., Duan, S. et al. YTHDF2 orchestrates tumor-associated macrophage reprogramming and controls antitumor immunity through CD8+ T cells. Nat Immunol 24, 255–266 (2023). https://doi.org/10.1038/s41590-022-01398-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-022-01398-6
This article is cited by
-
Macrophage barrier in the tumor microenvironment and potential clinical applications
Cell Communication and Signaling (2024)
-
METTL3 and METTL14-mediated N6-methyladenosine modification of SREBF2-AS1 facilitates hepatocellular carcinoma progression and sorafenib resistance through DNA demethylation of SREBF2
Scientific Reports (2024)
-
Fe-doped carbon dots: a novel biocompatible nanoplatform for multi-level cancer therapy
Journal of Nanobiotechnology (2023)
-
The Notch signaling pathway: a potential target for cancer immunotherapy
Journal of Hematology & Oncology (2023)
-
TRAF3/STAT6 axis regulates macrophage polarization and tumor progression
Cell Death & Differentiation (2023)