Abstract
Metastasis is the leading cause of cancer-related deaths and myeloid cells are critical in the metastatic microenvironment. Here, we explore the implications of reprogramming pre-metastatic niche myeloid cells by inducing trained immunity with whole beta-glucan particle (WGP). WGP-trained macrophages had increased responsiveness not only to lipopolysaccharide but also to tumor-derived factors. WGP in vivo treatment led to a trained immunity phenotype in lung interstitial macrophages, resulting in inhibition of tumor metastasis and survival prolongation in multiple mouse models of metastasis. WGP-induced trained immunity is mediated by the metabolite sphingosine-1-phosphate. Adoptive transfer of WGP-trained bone marrow-derived macrophages reduced tumor lung metastasis. Blockade of sphingosine-1-phosphate synthesis and mitochondrial fission abrogated WGP-induced trained immunity and its inhibition of lung metastases. WGP also induced trained immunity in human monocytes, resulting in antitumor activity. Our study identifies the metabolic sphingolipid–mitochondrial fission pathway for WGP-induced trained immunity and control over metastasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
RNA-seq data are available at the GEO under accession GSE195750. Source data are provided with this paper.
References
Welch, D. R. & Hurst, D. R. Defining the hallmarks of metastasis. Cancer Res 79, 3011–3027 (2019).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Kaplan, R. N., Rafii, S. & Lyden, D. Preparing the ‘soil’: the premetastatic niche. Cancer Res. 66, 11089–11093 (2006).
Morrissey, S. M. et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 33, 2040–2058 (2021).
Kaczanowska, S. et al. Genetically engineered myeloid cells rebalance the core immune suppression program in metastasis. Cell 184, 2033–2052 (2021).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
Netea, M. G., Quintin, J. & van der Meer, J. W. Trained immunity: a memory for innate host defense. Cell Host Microbe 9, 355–361 (2011).
Netea, M. G. et al. Trained immunity: a program of innate immune memory in health and disease. Science 352, aaf1098 (2016).
Saeed, S. et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345, 1251086 (2014).
Cheng, S. C. et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345, 1250684 (2014).
Divangahi, M. et al. Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat. Immunol. 22, 2–6 (2021).
Quintin, J. et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12, 223–232 (2012).
Li, X. et al. Maladaptive innate immune training of myelopoiesis links inflammatory comorbidities. Cell 185, 1709–1727 (2022).
Ziogas, A. & Netea, M. G. Trained immunity-related vaccines: innate immune memory and heterologous protection against infections. Trends Mol. Med 28, 497–512 (2022).
Kalafati, L. et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell 183, 771–785 (2020).
van Puffelen, J. H. et al. Trained immunity as a molecular mechanism for BCG immunotherapy in bladder cancer. Nat. Rev. Urol. 17, 513–525 (2020).
Stothers, C. L. et al. Beta-glucan induces distinct and protective innate immune memory in differentiated macrophages. J. Immunol. 207, 2785–2798 (2021).
Qi, C. et al. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived beta-glucans. Blood 117, 6825–6836 (2011).
Liu, M. et al. Transcription factor c-Maf is a checkpoint that programs macrophages in lung cancer. J. Clin. Invest 130, 2081–2096 (2020).
Tian, J. et al. Beta-glucan enhances antitumor immune responses by regulating differentiation and function of monocytic myeloid-derived suppressor cells. Eur. J. Immunol. 43, 1220–1230 (2013).
Li, W., Wang, H., Xu, X. G. & Yu, Y. Simultaneous nanoscale imaging of chemical and architectural heterogeneity on yeast cell wall particles. Langmuir 36, 6169–6177 (2020).
Wang, L. et al. Nanoscale simultaneous chemical and mechanical imaging via peak force infrared microscopy. Sci. Adv. 3, e1700255 (2017).
Noe, J. T. & Mitchell, R. A. MIF-dependent control of tumor immunity. Front. Immunol. 11, 609948 (2020).
Leng, L. et al. MIF signal transduction initiated by binding to CD74. J. Exp. Med. 197, 1467–1476 (2003).
Mitroulis, I. et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172, 147–161 (2018).
Khan, N. et al. M. tuberculosis reprograms hematopoietic stem cells to limit myelopoiesis and impair trained immunity. Cell 183, 752–770 (2020).
Kaufmann, E. et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172, 176–190 (2018).
Chakarov, S. et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363, eaau0964 (2019).
Schyns, J. et al. Non-classical tissue monocytes and two functionally distinct populations of interstitial macrophages populate the mouse lung. Nat. Commun. 10, 3964 (2019).
Gibbings, S. L. et al. Three unique interstitial macrophages in the murine lung at steady state. Am. J. Respir. Cell Mol. Biol. 57, 66–76 (2017).
Sánchez-Ramón, S. et al. Trained immunity-based vaccines: a new paradigm for the development of broad-spectrum anti-infectious formulations. Front. Immunol. 9, 2936 (2018).
Zhang, Z. et al. Differential expression of FAK and Pyk2 in metastatic and non-metastatic EL4 lymphoma cell lines. Clin. Exp. Metastasis 28, 551–565 (2011).
Satpathy, S. R. et al. Crystalline silica-induced leukotriene B4-dependent inflammation promotes lung tumour growth. Nat. Commun. 6, 7064 (2015).
Dominguez-Andres, J., Joosten, L. A. & Netea, M. G. Induction of innate immune memory: the role of cellular metabolism. Curr. Opin. Immunol. 56, 10–16 (2018).
Ciarlo, E. et al. Trained immunity confers broad-spectrum protection against bacterial infections. J. Infect. Dis. 222, 1869–1881 (2020).
Akbal, A. et al. How location and cellular signaling combine to activate the NLRP3 inflammasome. Cell Mol. Immunol. 19, 1201–1214 (2022).
Fugio, L. B., Coeli-Lacchini, F. B. & Leopoldino, A. M. Sphingolipids and mitochondrial dynamic. Cells 9, 581 (2020).
Brand, C. S., Tan, V. P., Brown, J. H. & Miyamoto, S. RhoA regulates Drp1 mediated mitochondrial fission through ROCK to protect cardiomyocytes. Cell. Signal. 50, 48–57 (2018).
Priem, B. et al. Trained immunity-promoting nanobiologic therapy suppresses tumor growth and potentiates checkpoint inhibition. Cell 183, 786–801 (2020).
Redig, A. J. & McAllister, S. S. Breast cancer as a systemic disease: a view of metastasis. J. Intern. Med. 274, 113–126 (2013).
Kotsakis, A. et al. Epidemiological characteristics, clinical outcomes and management patterns of metastatic breast cancer patients in routine clinical care settings of Greece: results from the EMERGE multicenter retrospective chart review study. BMC Cancer 19, 88 (2019).
Barton, M. K. Earlier adjuvant therapy is beneficial in patients with breast and colon cancer. CA Cancer J. Clin. 66, 3–5 (2016).
Goodridge, H. S. et al. Activation of the innate immune receptor dectin-1 upon formation of a ‘phagocytic synapse’. Nature 472, 471–475 (2011).
Finck, A. V., Blanchard, T., Roselle, C. P., Golinelli, G. & June, C. H. Engineered cellular immunotherapies in cancer and beyond. Nat. Med. 28, 678–689 (2022).
Camilli, G. et al. Beta-glucan-induced reprogramming of human macrophages inhibits NLRP3 inflammasome activation in cryopyrinopathies. J. Clin. Invest. 130, 4561–4573 (2020).
Bekkering, S. et al. Metabolic induction of trained immunity through the mevalonate pathway. Cell 172, 135–146 (2018).
Bekkering, S. et al. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 34, 1731–1738 (2014).
Weigert, A., Olesch, C. & Brüne, B. Sphingosine-1-phosphate and macrophage biology—how the sphinx tames the big eater. Front. Immunol. 10, 1706 (2019).
Groh, L. A. et al. oxLDL-induced trained immunity is dependent on mitochondrial metabolic reprogramming. Immunometabolism 3, e210025 (2021).
Geller, A. E. et al. The induction of peripheral trained immunity in the pancreas incites anti-tumor activity to control pancreatic cancer progression. Nat. Commun. 13, 759 (2022).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Subramanian, A. et al. Gene-set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959).
Acknowledgements
The authors thank W. Zacharias, M. Zhang and S. Waigel from the Brown Cancer Center Genomics Facility for their help in RNA-seq. GFP-dectin-1- expressing RAW 264.7 cells were kindly provided by D. Underhill from Cedars-Sinai Medical Center. This work was supported by the NIH grants R01CA213990 and R01AI128818 (to J.Y.). C.D., D.T. and E.C.R. were supported in part by the NIH grant P20GM135004. W.L. and Y.Y. were supported by NIH grant R35GM124918. H.W. and X.G.X. were supported by NSF CHE 1847765 and a Beckman Young Investigator Award from the Arnold and Mabel Beckman Foundation. Part of this work was performed with assistance of the UofL Genomics Facility, which is supported by NIH P20GM103436 (KY IDeA Networks of Biomedical Research Excellence), the Brown Cancer Center and user fees. Sequencing and bioinformatics support for this work was provided by NIH P20GM103436 (N. Cooper, Principal Investigator) and NIH P20GM106396 (D. Miller, Principal Investigator). The contents of this work are solely the responsibility of the authors and do not represent the official views of the NIH or the National Institute for General Medical Sciences (NIGMS).
Author information
Authors and Affiliations
Contributions
C.D. and R.S. designed and performed the experiments, analyzed data and interpreted results. R.S. and J.Y. wrote the manuscript. X.Z., A.E.G. and M.W. performed supporting experiments. R.M., H.-G.Z., L.S., X.Z., Y.Y. and X.G.X contributed to experimental design, data acquisition and data interpretation. W.L. and H.W. performed WGP characterization and phagocytosis imaging experiments. F.Y. performed and analyzed S1P data by LC–MS/MS. H.L. performed CyTOF experiments, and H.L. and D.T. performed CyTOF data analysis. J.H.C. and E.C.R. performed computational analysis and assisted in interpretation of RNA-seq data. R.M., L.S., X.Z., K.M.M. and Y.Y. provided materials and contributed to a critical review of the project. J.Y. directed experimental design and the overall study, interpreted data, supervised research and performed a final review of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Maziar Divangahi, Markus Maeurer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: N. Bernard, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 WGP characterization.
a, Topography image and PFIR images at 1040 cm−1 of a dried WGP particle on silica substrate. b, PFIR spectra scan at 4 different spots marked in the zoom-in topography image. c, Stiffness and adhesion images of the same dried WGP particle. Scale bars: 2 μm; scale bars in zoom-in images: 500 nm. d, Phagocytosis process of WGP by GFP-Dectin-1 (green) RAW 264.7 macrophage. Dectin-1 was recruited and clustered at the site of phagocytic cups. Scale bars: 20 μm. e, Fluorescence confocal microscopy images (in maximum projection) showing heterogeneous recruitment of Dectin-1 receptors to phagosomes. Scale bars: 10 μm.
Extended Data Fig. 2 WGP mediates a systemic increase of trained macrophages.
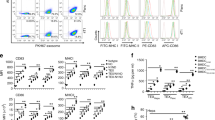
a, Frequency of LSK cells (Lin−c-Kit+Sca-1+) and b, frequency of multi-potent progenitors (MPPs) (Lin−cKit+Sca1+CD150−CD48+) in the BM of PBS (n = 7) vs WGP-trained (n = 9) mice. Representative dot plots and summarized data from two independent experiments are shown. c, Frequency of CD11b+ myeloid cells in the spleen (n = 9-10) and lymph nodes (n = 7). Representative dot plots and pooled of two independent experiments are shown. d, Intracellular TNF levels on F4/80+ macrophages in the spleen and lymph node from PBS (n = 4) vs WGP-trained (n = 6) mice after ex vivo re-stimulation with LPS. Representative dot plots and summarized percent of TNF-α+ macrophages and MFI are shown. Data are representative of two independent experiments. e, Representative images for histology of the lungs from PBS, 24 h and 7 days post WGP treatment. Scale bar = 500μm. f, Gating strategy for Lung AM and IM. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. P values were derived from unpaired two-tailed student’s t-test for a, b, d, e; one-way ANOVA with Tukey’s multiple comparison test for f, g.
Extended Data Fig. 3 Lung IMs bear a trained immunity upon WGP in vivo treatment.
a, CCR2 expression on lung AMs and IMs from naïve WT C57Bl/6 mice assessed by flow cytometry. b, Mice were trained with (n = 5) or without WGP (n = 4) (1 mg IP on day 0) and euthanized at day 7. Frequency of CCR2+ and CCR2− IMs was determined by flow cytometry. Representative dot plots and summarized data are shown. c, Both CCR2+ and CCR2− IMs display a trained immunity phenotype. Lung cells from WGP-trained or control mice were restimulated with LPS and intracellular TNF production was assessed by flow cytometry. Cells were gated on CCR2+ or CCR2− IMs. d, Summarized data ofpooled two independent experiments lung AMs and IMs from WT (n = 8) and CCR2 KO (n = 8) mice. e, WT and CCR2 KO mice were trained with or without WGP (n = 4). Lung cells were restimulated with LPS. Intracellular TNF was determined by flow cytometry. Representative dot plots and summarized percent and MFI data are shown. f, Mice were IP administered with PBS or WGP (0.5 mg) or WGP (2 mg) and analyzed for the lung IM phenotype at day 7 (n = 5). Frequency and MFI for intracellular TNF expression on lung IM after ex vivo re-stimulation with LPS were determine by flow cytometry. Representative dot plots and summarized percent and MFI data are shown. g, Mice were injected IP with PBS (n = 6) or WGP (1 mg/mouse, n = 6) or polystyrene beads (1 mg, n = 5) and analyzed for the lung IM phenotype at day 7. Frequency and MFI for intracellular TNF expression on lung IM after ex vivo re-stimulation with LPS. Representative dot plots and summarized percent and MFI data are shown. Data are representative as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****p < 0.0001. P values were derived from one-way ANOVA with Tukey’s multiple comparison test.
Extended Data Fig. 4 WGP-induced training modulates T cells and BM progenitors.
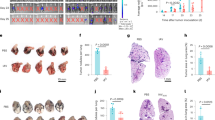
a, PBS (n = 9) or WGP-trained (n = 10) mice were challenged with 0.4 × 106 LLC cells intravenously. Mice were enthunized at day 16 for T cell phenotype analysis. Percentage of FoxP3+ Tregs, TNF expressing CD4+ and CD8+ T cells in the lungs from PBS vs WGP-trained mice. Data are representative of two independent experiments pooled together. b, Mice were trained with or without WGP (n = 5) for 7 days and then challenged with 0.4 × 106 EL4 lymphoma cells i.v. followed by euthanizing at day 16 post tumor cell injection. Representative liver micrographs, summarized number of liver nodules, and liver weights of PBS and WGP-trained EL4 tumor cell-bearing mice are shown. c, CCR2 expression on lung IMs and intracellular TNF production in CCR2+ IMs from mice reconstituted with BM cells from WGP-trained or PBS control mice (n = 5). Representative dot plots and summarized data from one of two independent experiments are shown. Data are presented as mean ± SEM. **P < 0.01, ***P < 0.001, ****P < 0.0001. P values were derived from unpaired two-tailed student’s t-test for a, b and c.
Extended Data Fig. 5 Macrophages are the effector cells that control metastasis.
a, Depletion efficiency of lung IM and spleenic macrophages after Clodronate liposome (Clodrosome) injection. b, Summarized data for percentage of LLC-GFP cells in WGP-trained and untrained WT and CCR2 KO mice. WT and CCR2 KO mice were trained with PBS or WGP for a week followed by challenge with i.v. GFP-LLC cells (n = 5). Mice were then euthanized to analyze the tumor burden in the lungs 2 weeks later by flow cytometry. c, Schema for in vivo Ly6G depletion, WGP training, and tumor challenge protocol (n = 5, 5, 4, 5). d, Depletion efficiency of lung Ly6G+ neutrophils after weekly injections with Ly6G depletion antibody or isotype antibody by flow cytometry. e, Summarized data for percentage of LLC-GFP cells in neutrophil depleted or isotype antibody treated WGP trained and untrained mice. f, Depletion efficiency of CD4 and CD8 T cells in the lungs after CD4 and CD8 depletion antibody or isotype antibody injection. g, Representative dot plots and summarized data for intracellular TNF expression on lung IM after ex vivo LPS re-stimulation of PBS versus WGP-trained NSG mice (n = 4). h, Representative dot plots and summarized data for tumor burden in the lungs of WGP trained and untrained NSG mice. NSG mice trained with PBS (n = 7) or WGP (n = 9) were challenged with GFP-LLC cells i.v. and the lungs were harvested 48 h later to analyze the tumor burden in the lungs by flow cytometry. Data are representative of one of two independent experiments and presented as mean ± SEM. **P < 0.01, ***P < 0.001, ****P < 0.0001. P values were derived from two way ANOVA with Tukey’s multiple comparison test for b, one way ANOVA with Tukey’s multiple comparison test for e, unpaired two-tailed student’s t-test for g and h.
Extended Data Fig. 6 Trained lung IMs express DEGs related to innate function.
RNAseq was performed on lung IM sorted from PBS and WGP-trained mice (n = 3) on day 7. a, Volcano plot shows differentially expressed genes (DEGs) in WGP-trained lung IMs compared to PBS controls. Cers6 gene is among the top upregulated DEGs in WGP-trained lung IMs (circled). b, GO pathway analysis shows enriched pathways in WGP-trained lung IMs. c, GSEA plots show enriched differential pathways in WGP-trained lung IMs. DESeq2 for differential expression analysis was used for statistical analysis (a, b).
Extended Data Fig. 7 WGP-mediated training is independent of mTOR/HIF-1α or IL-1β.
a, TNF production by WGP-trained peritoneal macrophages sorted from Raptor cKO or control mice after LPS re-stimulation (n = 2). Data representative of one of three independent experiments. b, Percentage of LLC-GFP cells in the lungs from PBS vs WGP-trained control and Raptor cKO mice (n = 3, 3, 5, 7). Representative dot plots and summarized data for one of two independent experiments are shown. c, TNF production by WGP-trained peritoneal macrophages sorted from HIF-1α cKO or control mice after LPS re-stimulation (n = 3). Data representative of one of three independent experiments. d, Percentage of LLC-GFP cells in the lungs from PBS vs WGP-trained control and HIF-1α cKO mice (n = 5, 5, 11, 10). Representative dot plots and summarized data for two independent experiments pooled together are shown. e, TNF production by WGP-trained or untrained peritoneal macrophages sorted from IL-1R KO mice after LPS re-stimulation (left) (n = 4) and percentage of LLC-GFP cells in the lungs from PBS (n = 4) or WGP-trained IL-1R KO (n = 5) mice (right). f, TNF production by WGP-trained peritoneal macrophages sorted from WT and Nlrp3 KO mice after LPS re-stimulation (n = 4). Data are representative of one of two independent experiments and presented as mean ± SEM. **P < 0.01, ***P < 0.001, ****P < 0.0001. P values were derived from two-way ANOVA with Tukey’s multiple comparison test for a, c; one-way ANOVA with Tukey’s multiple comparison test for b, d, e, f.
Extended Data Fig. 8 WGP induces p-Drp-1 and mitochondrial fission in macrophages.
a, Summarized data for western blot of p-Drp-1 in peritoneal macrophages stimulated with WGP for different time points in the presence or absence of Sphk2i or DMSO vehicle control (n = 3). Data representative of three independent experiments pooled together. b, Representative images for Transmission Electron Microscopy (TEM) of untrained and WGP-trained peritoneal macrophages (left) and summarized data for mitochondrial lengths (right) (n = 100). Data are presented as mean ± SEM. **P < 0.01, ***P < 0.001, ****P < 0.0001. P values were derived from one-way ANOVA with Tukey’s multiple comparison test for a, chi-square test for b.
Extended Data Fig. 9 Mitochondrial fission is critical in WGP-induced trained immunity.
Mice were trained with PBS or WGP along with DMSO or Mdivi-1 daily treatment for 6 days (n = 5, 4, 5, 5). a, Summarized percentages of LSK+ cells (left) and MPPs (right) in the BM. b, Summarized percentages of lung total CD11b+ myeloid cells and IMs. Data are representative of one of two independent experiments. c, Intracellular TNF expression on lung IMs from PBS vs WGP-trained, DMSO vs Mdivi-1-treated mice. Representative dot plots and summarized data are shown. d, Representative tSNE plots show TNF production by CD4 and CD8 T cells in mice treated with different regimens. e, Schema for in vivo training with PBS or WGP in the presence of Sphk2 inhibitor or DMSO vehicle control and tumor challenge protocol (n = 9, 10, 9, 9). f, Representative dot plots and summarized data for percentage of GFP-LLC in the lungs. Mice were treated with Sphk2 inhibitor or vehicle control DMSO (50 mg/kg, i.p.) followed by PBS or WGP administration i.p. 2-3 h later. Treatment with Sphk2i or DMSO were performed daily until D5 followed by challenge with GFP-LLC i.v. Tumor burden in the lungs on D16 post-tumor challenge was analyzed. Data are pooled oftwo independent experiments. g, Schema for therapeutic model of BMDM adoptive transfer. 6 weeks old female C57Bl/6 mice were challenged with 0.4 × 106 LLC-GFP tumor cells followed by two adoptive transfer of untrained or WGP-trained BMDM at D3 and D6 (n = 7). Mice were then euthanized at D16 to analyze the tumor burden in the lungs. h, Representative dot plots and summarized percentages for tumor burden in the lungs shown. Data are representative of one of two independent experiments and presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. P values were derived from one-way ANOVA with Tukey’s multiple comparison test for a, b, c, f and h.
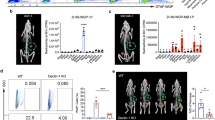
Extended Data Fig. 10 WGP induces trained immunity in human monocytes.
a, Human CD14+ monocytes from healthy donors (n = 4) were trained with WGP in vitro and then restimulated with LPS 7 days later. Culture supernatants were assayed for TNF and IL-6 by ELISA. b, WGP-trained monocytes were re-stimulated with culture supernatants from A549 or HBEC. TNF and IL-6 were measured by ELISA. c, WGP-induced mitochondrial ROS assessed by MitoSox staining (n = 6, 11). d, Western blot analysis for phosphorylation of Drp-1 (p-Drp-1 (n = 3). e, Flow cytometry analysis for p-Drp1 in control vs WGP-trained monocytes (n = 2). f, TEM images for untrained and WGP-trained monocytes. Representative images and summarized data for mitochondrial lengths (n = 100) are shown. g, Intracellular S1P quantitation in untrained vs WGP-trained human monocytes by LC/MS/MS. h, S1P-induced mitochondrial ROS assessed by Mitosox staining (n = 7, 8). i, Flow cytometric analysis of p-Drp1 for S1P-treated human monocytes (n = 4). j, Human monocytes trained with WGP were re-stimulated with LPS in the presence or absence of Mdivi-1. TNF production was measured by ELISA (n = 4). k, Human monocytes trained with or without WGP were co-cultured with luciferase+ A549 lung cancer cells at 10:1 and 20:1 ratios for 12–16 h (n = 5). Cytotoxicity was assessed as a measure of luciferase activity using a luminometer. l, Bioluminescence imaging (BLI) of NSG mice with orthotopic A549-luciferase tumor admixed with control (n = 6) vs WGP-trained human CD14+ (n = 9) monocytes. Data in c, d, and l are poold from two to three independent experiments . Data in a, b, e, g, h, i, j, and k are representative of two or three independent experiments. All data are presented as mean ± SEM. **P < 0.01, ***P < 0.001, ****P < 0.0001.P values were derived from two-way ANOVA with Tukey’s multiple comparison test for a, b, j, k; unpaired two-tailed student’s t-test for c, d, e, g, h, i, l; chi-square test for f.
Supplementary information
Source data
Source Data Fig. 1
Source data.
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Source data.
Source Data Fig. 3
Source data.
Source Data Fig. 4
Source data.
Source Data Fig. 5
Source data.
Source Data Fig. 6
Source data.
Source Data Fig. 7
Source data.
Source Data Fig. 7
Unprocessed western blots.
Source Data Fig. 8
Source data.
Source Data Extended Data Fig. 2
Source data.
Source Data Extended Data Fig. 3
Source data.
Source Data Extended Data Fig. 4
Source data.
Source Data Extended Data Fig. 5
Source data.
Source Data Extended Data Fig. 7
Source data.
Source Data Extended Data Fig. 8
Source data.
Source Data Extended Data Fig. 9
Source data.
Source Data Extended Data Fig. 10
Source data.
Source Data Extended Data Fig.10
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, C., Shrestha, R., Zhu, X. et al. Inducing trained immunity in pro-metastatic macrophages to control tumor metastasis. Nat Immunol 24, 239–254 (2023). https://doi.org/10.1038/s41590-022-01388-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-022-01388-8
This article is cited by
-
Mitochondrial dynamics and colorectal cancer biology: mechanisms and potential targets
Cell Communication and Signaling (2024)
-
Kupffer cells prevent pancreatic ductal adenocarcinoma metastasis to the liver in mice
Nature Communications (2023)
-
A novel role for mitochondrial fission in macrophages: trained innate immunity induced by beta-glucan
Cellular & Molecular Immunology (2023)