Abstract
Expressed on epidermal Langerhans cells, CD1a presents a range of self-lipid antigens found within the skin; however, the extent to which CD1a presents microbial ligands from bacteria colonizing the skin is unclear. Here we identified CD1a-dependent T cell responses to phosphatidylglycerol (PG), a ubiquitous bacterial membrane phospholipid, as well as to lysylPG, a modified PG, present in several Gram-positive bacteria and highly abundant in Staphylococcus aureus. The crystal structure of the CD1a–PG complex showed that the acyl chains were buried within the A′- and F′-pockets of CD1a, while the phosphoglycerol headgroup remained solvent exposed in the F′-portal and was available for T cell receptor contact. Using lysylPG and PG-loaded CD1a tetramers, we identified T cells in peripheral blood and in skin that respond to these lipids in a dose-dependent manner. Tetramer+CD4+ T cell lines secreted type 2 helper T cell cytokines in response to phosphatidylglycerols as well as to co-cultures of CD1a+ dendritic cells and Staphylococcus bacteria. The expansion in patients with atopic dermatitis of CD4+ CD1a–(lysyl)PG tetramer+ T cells suggests a response to lipids made by bacteria associated with atopic dermatitis and provides a link supporting involvement of PG-based lipid-activated T cells in atopic dermatitis pathogenesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The CD1a-PG final model and structure factor files were deposited in the PDB under ID 7SH4. Single-cell and bulk RNA-seq files are available in the Gene Expression Omnibus under accession no. GSE186459.Source data are provided with this paper.
Code availability
The Seurat object (RDS file format), intermediate output files and differential expression files (csv format) along with the code used in this manuscript for single-cell and bulk RNA-seq analysis is available in GitHub project repository (https://github.com/IshaMonga/CD1a-single-cell).
References
Murphy, G. F., Bhan, A. K., Sato, S., Mihm, M. C. Jr & Harrist, T. J. A new immunologic marker for human Langerhans cells. N. Engl. J. Med. 304, 791–792 (1981).
de Fraissinette, A., Schmitt, D. & Thivolet, J. Langerhans cells of human mucosa. J. Dermatol. 16, 255–262 (1989).
Kubo, A., Nagao, K., Yokouchi, M., Sasaki, H. & Amagai, M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 206, 2937–2946 (2009).
Byrd, A. L., Belkaid, Y. & Segre, J. A. The human skin microbiome. Nat. Rev. Microbiol. 16, 143–155 (2018).
Moody, D. B. et al. T cell activation by lipopeptide antigens. Science 303, 527–531 (2004).
Kasmar, A. G. et al. Cutting edge: CD1a tetramers and dextramers identify human lipopeptide-specific T cells ex vivo. J. Immunol. 191, 4499–4503 (2013).
Ouchi, T. et al. Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome. J. Exp. Med. 208, 2607–2613 (2011).
Sohlenkamp, C. & Geiger, O. Bacterial membrane lipids: diversity in structures and pathways. FEMS Microbiol. Rev. 40, 133–159 (2016).
Dugail, I., Kayser, B. D. & Lhomme, M. Specific roles of phosphatidylglycerols in hosts and microbes. Biochimie 141, 47–53 (2017).
Peschel, A. et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193, 1067–1076 (2001).
Slavetinsky, C., Kuhn, S. & Peschel, A. Bacterial aminoacyl phospholipids—biosynthesis and role in basic cellular processes and pathogenicity. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 1862, 1310–1318 (2017).
Ernst, C. M. & Peschel, A. MprF-mediated daptomycin resistance. Int. J. Med. Microbiol. 309, 359–363 (2019).
Kuhn, S., Slavetinsky, C. J. & Peschel, A. Synthesis and function of phospholipids in Staphylococcus aureus. Int. J. Med. Microbiol. 305, 196–202 (2015).
Hines, K. M. et al. Lipidomic and ultrastructural characterization of the cell envelope of Staphylococcus aureus grown in the presence of human serum. mSphere 5, e00339–20 (2020).
Grice, E. A. et al. Topographical and temporal diversity of the human skin microbiome. Science 324, 1190–1192 (2009).
Morita, S. Y. & Terada, T. Enzymatic measurement of phosphatidylglycerol and cardiolipin in cultured cells and mitochondria. Sci. Rep. 5, 11737 (2015).
de Jong, A. et al. CD1a-autoreactive T cells are a normal component of the human αβ T cell repertoire. Nat. Immunol. 11, 1102–1109 (2010).
Cotton, R. N. et al. Human skin is colonized by T cells that recognize CD1a independently of lipid. J. Clin. Investig. 131, e140706 (2021).
de Jong, A. et al. CD1a-autoreactive T cells recognize natural skin oils that function as headless antigens. Nat. Immunol. 15, 177–185 (2014).
Birkinshaw, R. W. et al. αβ T cell antigen receptor recognition of CD1a presenting self lipid ligands. Nat. Immunol. 16, 258–266 (2015).
de Lalla, C. et al. High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. Eur. J. Immunol. 41, 602–610 (2011).
Danner, S., Pabst, G., Lohner, K. & Hickel, A. Structure and thermotropic behavior of the Staphylococcus aureus lipid lysyl-dipalmitoylphosphatidylglycerol. Biophys. J. 94, 2150–2159 (2008).
Hashimoto, K. et al. Single-cell transcriptomics reveals expansion of cytotoxic CD4 T cells in supercentenarians. Proc. Natl Acad. Sci. USA 116, 24242–24251 (2019).
Takeuchi, A. et al. CRTAM determines the CD4+ cytotoxic T lymphocyte lineage. J. Exp. Med. 213, 123–138 (2016).
Takeuchi, A. & Saito, T. CD4 CTL, a cytotoxic subset of CD4(+) T cells, their differentiation and function. Front. Immunol. 8, 194 (2017).
Bush, E. C. et al. PLATE-seq for genome-wide regulatory network analysis of high-throughput screens. Nat. Commun. 8, 105 (2017).
Patil, V.S. et al. Precursors of human CD4(+) cytotoxic T lymphocytes identified by single-cell transcriptome analysis. Sci. Immunol. 3, eaan8664 (2018).
Szabo, P. A. et al. Single-cell transcriptomics of human T cells reveals tissue and activation signatures in health and disease. Nat. Commun. 10, 4706 (2019).
Cano-Gamez, E. et al. Single-cell transcriptomics identifies an effectorness gradient shaping the response of CD4(+) T cells to cytokines. Nat. Commun. 11, 1801 (2020).
Gutierrez-Arcelus, M. et al. Lymphocyte innateness defined by transcriptional states reflects a balance between proliferation and effector functions. Nat. Commun. 10, 687 (2019).
Zheng, W. & Flavell, R. A. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89, 587–596 (1997).
Flavell, R. A. et al. Molecular basis of T-cell differentiation. Cold Spring Harb. Symp. Quant. Biol. 64, 563–571 (1999).
Thornton, A. M. et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 184, 3433–3441 (2010).
Gregg, R. et al. The number of human peripheral blood CD4+CD25high regulatory T cells increases with age. Clin. Exp. Immunol. 140, 540–546 (2005).
Leyden, J. J., Marples, R. R. & Kligman, A. M. Staphylococcus aureus in the lesions of atopic dermatitis. Br. J. Dermatol. 90, 525–530 (1974).
Kong, H. H. et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 22, 850–859 (2012).
De Benedetto, A. et al. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 127, 773–786 (2011).
Bos, J. D. et al. Predominance of ‘memory’ T cells (CD4+, CDw29+) over ‘naive’ T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch. Dermatol. Res. 281, 24–30 (1989).
Guttman-Yassky, E. et al. Major differences in inflammatory dendritic cells and their products distinguish atopic dermatitis from psoriasis. J. Allergy Clin. Immunol. 119, 1210–1217 (2007).
Van Rhijn, I. et al. Human autoreactive T cells recognize CD1b and phospholipids. Proc. Natl Acad. Sci. USA 113, 380–385 (2016).
Shahine, A. et al. A molecular basis of human T cell receptor autoreactivity toward self-phospholipids. Sci. Immunol. 2, eaao1384 (2017).
Shahine, A. et al. A T-cell receptor escape channel allows broad T-cell response to CD1b and membrane phospholipids. Nat. Commun. 10, 56 (2019).
Wolf, B. J. et al. Identification of a potent microbial lipid antigen for diverse NKT cells. J. Immunol. 195, 2540–2551 (2015).
Tatituri, R. V. et al. Recognition of microbial and mammalian phospholipid antigens by NKT cells with diverse TCRs. Proc. Natl Acad. Sci. USA 110, 1827–1832 (2013).
Visvabharathy, L. et al. Group 1 CD1-restricted T cells contribute to control of systemic Staphylococcus aureus infection. PLoS Pathog. 16, e1008443 (2020).
Zajonc, D. M. et al. Molecular mechanism of lipopeptide presentation by CD1a. Immunity 22, 209–219 (2005).
Cotton, R.N. et al. CD1a selectively captures endogenous cellular lipids that broadly block T cell response. J. Exp. Med. 218, e20202699 (2021).
Veldhuizen, R., Nag, K., Orgeig, S. & Possmayer, F. The role of lipids in pulmonary surfactant. Biochim. Biophys. Acta 1408, 90–108 (1998).
Clark, R. A. et al. A novel method for the isolation of skin resident T cells from normal and diseased human skin. J. Invest. Dermatol. 126, 1059–1070 (2006).
Kabsch, W. XDS. Acta Crystallogr. D 66, 125–132 (2010).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D 67, 235–242 (2011).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D 68, 352–367 (2012).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959).
van ‘t Klooster, J. S. et al. Periprotein lipidomes of Saccharomyces cerevisiae provide a flexible environment for conformational changes of membrane proteins. eLife 9, e57003 (2020).
Lun, A. T. L. & Marioni, J. C. Overcoming confounding plate effects in differential expression analyses of single-cell RNA-seq data. Biostatistics 18, 451–464 (2017).
Snyder, M. E. et al. Generation and persistence of human tissue-resident memory T cells in lung transplantation. Sci. Immunol. 4, eaav5581 (2019).
Granot, T. et al. Dendritic cells display subset and tissue-specific maturation dynamics over human life. Immunity 46, 504–515 (2017).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Osorio, D. & Cai, J. J. Systematic determination of the mitochondrial proportion in human and mice tissues for single-cell RNA-sequencing data quality control. Bioinformatics 37, 963–967 (2021).
Acknowledgements
We thank the NIH Tetramer Core Facility for CD1 proteins, P. Sims and M. Finlayson for their advice regarding the analysis of single-cell RNA-seq data, A. Peschel for providing the S. aureus strain, Q. Cremers for assistance with ChemDraw and I. van Rhijn for critical reading of the manuscript. The work is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR074037, K01 AR068475 and P30 AR069632 to A.d.J. and R01 AR048632 to D.B.M.) and the Wellcome Trust Collaborative Award (to D.B.M., G.O. and J.R.) as well as an Irving Scholarship (to A.d.J.). J.R. is supported by a National Health and Medical Research Council Investigator award. G.O. receives funding from the Medical Research Council UK and National Institute for Health and Care Research Oxford Biomedical Research Centre. This work was supported by the NCI Cancer Center Support Grant (P30 CA013696), used the Genomics and High Throughput Screening Shared Resource and was also supported by the National Center for Advancing Translational Sciences (UL1 TR001873), as well as funding to Columbia University Irving Medical Center flow core facilities through S10RR027050 and S10OD020056. We thank the staff of the MX1 beamline of the Australian Synchrotron, part of Australian Nuclear Science and Technology.
Author information
Authors and Affiliations
Contributions
G.C.M. and A.D.J. conceived the project. G.C.M., R.C., I.M.K. and A.E.K. performed T cell and tetramer assays. G.C.M. and I.M. performed analysis of RNA-seq data and data submission. M.W., S.Y.T. and A.S. performed structural analysis and SPR studies. T.-Y.C. performed lipid elutions and quantification by HPLC–MS and A.H. and J.A.G.V. performed single-cell sequencing. B.N.S., L.N.U., S.Y., C.H.R. and L.A.B. recruited and enrolled study participants and collected clinical specimens. A.T. and A.C.U. provided cultured S. aureus and S. epidermidis for lipid analysis. Y.L.C., S.W.N. and G.O. completed tetramer analyses of the second patient cohort. B.C. performed statistical analyses. A.d.J., J.R. and D.B.M. provided oversight for experiments and input for the study. A.d.J. and G.C.M. prepared the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
A.d.J. and D.B.M. provide consulting to Pfizer. G.O. and Y.L.C. have relevant research collaborations with UCB and Janssen and a patent related to CD1a. The other authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Paolo Dellabona, Mikael Karlsson, Steven Porcelli and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: L. A. Dempsey, in collaboration with the Nature Immunology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
(a) Flow cytometric analysis of CD1a-autoreactive DermT cell line stained with the indicated CD1a tetramers: unloaded (endo), PC, PG and lysylPG, co-stained for CD4 and gated on live cells. Indicated in the dotplots is the percentage of tetramer+ T cells in the tetramer gate. (b) Electron density maps of phosphatidylglycerol C18:1, C18:0 in the cleft of CD1a. Left: Unbiased (green, Fo-Fc at 2.5 σ. Right: Refined (blue, 2Fo-Fc at 0.9 σ) (c) Co-receptor expression determined on CD1a tetramer+ T cells measured in Fig. 1f. Indicated are the percentages of tetramer+ T cells expressing CD4 (red), CD8 (blue), neither (white) of both (grey) of the co-receptors. Right graph: Percentage of CD4 + T cells among tetramer+ cells. *p < 0.05 as determined by Wilcoxon matched-pairs signed-rank test (2-sided).
Extended Data Fig. 2
(a) Three T cells lines containing CD1a-lysylPG tetramer+ T cells were staining with lysylPG-treated CD1b, CD1c and CD1d tetramers co-stained with anti-CD4 and analyzed by flow cytometry. Plots are gated on live T cells. (b) Human CD1/β2m isoforms were expressed in Expi293F GnTI- cells and purified by nickel-affinity and size exclusion chromatography. CD1 proteins carrying endogenous lipids (CD1-endo) were incubated with lysylPG 16 hrs at 25 °C in 0.1 M MES pH 5.5, 150 mM NaCl, 0.05% CHAPS. 1 µg of CD1-endo and CD1-lysylPG samples were loaded on a 3–9 gel (Cytiva), and displacement of endogenous lipid and/or shifts towards more electropositive values indicate efficient loading of lysylPG. (c) GM-CSF concentration in supernatant of CD1a-lysylPG tetramer+ T cell line (921a) after 24 h of incubation with plate-bound CD1a and indicated concentrations of PC, PG and lysylPG. Indicated are mean ± SD of triplicate values. P values were based on paired two-tailed t-test. * p < 0.05 ** p < 0.01.
Extended Data Fig. 3
(a) LysylPG was treated at the indicated pH at 25 °C overnight (16 hr) or 37 °C for 1 hr, followed by lipid extraction and HPLC–MS analysis. The PG and lysylPG were quantified as percentage of the sum of both lipids, based on the curve fitting of external standards. (b) Isoelectric focusing gel showing the migration pattern of human CD1a carrying heterogenous mixture of lipids derived from the mammalian expression system (CD1a-endo) upon incubation with lysylPG or PG at low (citrate pH 5.5) or high (tris pH 8) pH. Presence of negatively charged lipids (for example PG) in the cleft of CD1a shifts the PI of the protein towards electronegative values (CD1a−1). Degradation of lysylPG to PG at high pH results in migration pattern identical to CD1a-PG. (c) CD1a proteins treated with lysylPG (pH 5.5 or pH 8) described in (b) were extracted and the eluted lipids, lysylPG and PG were analyzed by HPLC–MS. (d) Isoelectric focusing gel showing distinct migration pattern of CD1a incubated with CHAPS, PG or lysylPG based on the pI of the protein. LysylPG-loaded CD1a was further incubated overnight in MMT buffer at different pH to assess the stability of the lysylated headgroup. The lack of band corresponding to more negatively charged species of CD1a (CD1a-1) suggests that lysylPG is stable once bound to CD1a.
Extended Data Fig. 4
FACS sorting of CD4 + CD1a-PG tetramer+ T cells from donor 211 PBMC. Sorted T cells were expanded in vitro for 14 days after which the cells were tested for specificity using dual tetramer staining with CD1a-PG (APC-labeled) and CD1a-lysylPG (PE-labeled).
Extended Data Fig. 5
(a) S. aureus PGs were saponified and methylated to form FAMEs. The bacterial C15 fatty acid was determined as branched by co-elution of its FAME with iso-C15 FAME and anteiso-C15 FAME external standards, but not with straight chain n-C15 FAME. Data are presented as an overlay of mass chromatograms of m/z. 257.2476 [C15 FAME + H]+ by the positive mode reversed-phase HPLC-QTOF-MS analysis. (b) CID_MS spectra for PG and lysylPG supports assignments in Fig. 6b.
Extended Data Fig. 6
(a, b) Mass chromatogram of eight most abundant PG and lysylPG species from S. aureus and S. epidermidis are depicted. (c) The detected PGs and lysylPGs by the QToF mass spectrometer are summarized. The quantity (ng) of each lipid species in injected total lipid extracts (5 µg) was determined by curve fitting of the chromatogram area to the external standard curve. The measurements were performed in triplicate (mean ± SD).
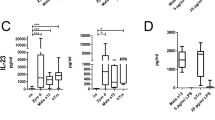
Extended Data Fig. 7 Supernatants from the IL-13 Elispot assays of DC – S. aureus/ S. epidermidis co-cultures with CD1a-(lysyl)PG tetramer+ CD4 + T cells (Fig. 6c) were analyzed by LEGENDplex in duplicate.
This panel measured 12 different human T cell cytokines, 11 of which are depicted (IL-13 was measured using Elispot and could therefore not be reliably measured in the supernatant). Concentrations in the supernatant for indicated cytokines are depicted for each culture condition (mean ± SD).
Extended Data Fig. 8 Three T cell lines (834, 325, 921a) and one T cell clone (2114.1) were stained with mock-treated, S.aureus PG-treated and C18:1 PG-treated CD1a tetramers.
The lines were co-stained with CD4 antibody and DAPI, and live cells were analyzed for tetramer staining by flow cytometry.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5, Table 1 and Notes.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed gel.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed gels.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Monnot, G.C., Wegrecki, M., Cheng, TY. et al. Staphylococcal phosphatidylglycerol antigens activate human T cells via CD1a. Nat Immunol 24, 110–122 (2023). https://doi.org/10.1038/s41590-022-01375-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-022-01375-z