Abstract
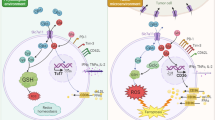
Amino acid metabolism is essential for cell survival, while the byproduct ammonia is toxic and can injure cellular longevity. Here we show that CD8+ memory T (TM) cells mobilize the carbamoyl phosphate (CP) metabolic pathway to clear ammonia, thus promoting memory development. CD8+ TM cells use β-hydroxybutyrylation to upregulate CP synthetase 1 and trigger the CP metabolic cascade to form arginine in the cytosol. This cytosolic arginine is then translocated into the mitochondria where it is split by arginase 2 to urea and ornithine. Cytosolic arginine is also converted to nitric oxide and citrulline by nitric oxide synthases. Thus, both the urea and citrulline cycles are employed by CD8+ T cells to clear ammonia and enable memory development. This ammonia clearance machinery might be targeted to improve T cell-based cancer immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data supporting this study are available within main article and Supplementary Information files or are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Lunt, S. Y. & Vander Heiden, M. G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464 (2011).
Mehta, M. M., Weinberg, S. E. & Chandel, N. S. Mitochondrial control of immunity: beyond ATP. Nat. Rev. Immunol. 17, 608–620 (2017).
Leone, R. D. & Powell, J. D. Metabolism of immune cells in cancer. Nat. Rev. Cancer 20, 516–531 (2020).
Zhang, J., Pavlova, N. N. & Thompson, C. B. Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. EMBO J. 36, 1302–1315 (2017).
Srinivas, U. S., Tan, B. W. Q., Vellayappan, B. A. & Jeyasekharan, A. D. ROS and the DNA damage response in cancer. Redox Biol. 25, 101084 (2019).
Li, L. et al. p53 regulation of ammonia metabolism through urea cycle controls polyamine biosynthesis. Nature 567, 253–256 (2019).
Dasarathy, S. et al. Ammonia toxicity: from head to toe? Metab. Brain Dis. 32, 529–538 (2017).
Sies, H. & Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21, 363–383 (2020).
Weiner, I. D., Mitch, W. E. & Sands, J. M. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin. J. Am. Soc. Nephrol. 10, 1444–1458 (2015).
Amsen, D., van Gisbergen, K., Hombrink, P. & van Lier, R. A. W. Tissue-resident memory T cells at the center of immunity to solid tumors. Nat. Immunol. 19, 538–546 (2018).
Kok, L., Masopust, D. & Schumacher, T. N. The precursors of CD8(+) tissue resident memory T cells: from lymphoid organs to infected tissues. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-021-00590-3 (2022).
Ma, R. et al. A Pck1-directed glycogen metabolic program regulates formation and maintenance of memory CD8(+) T cells. Nat. Cell Biol. 20, 21–27 (2018).
Zhang, H. et al. Ketogenesis-generated β-hydroxybutyrate is an epigenetic regulator of CD8(+) T-cell memory development. Nat. Cell Biol. 22, 18–25 (2020).
Wang, Y. et al. Coordinative metabolism of glutamine carbon and nitrogen in proliferating cancer cells under hypoxia. Nat. Commun. 10, 201 (2019).
Lercher, A. et al. Type I interferon signaling disrupts the hepatic urea cycle and alters systemic metabolism to suppress T cell function. Immunity 51, 1074–1087 (2019).
Keshet, R., Szlosarek, P., Carracedo, A. & Erez, A. Rewiring urea cycle metabolism in cancer to support anabolism. Nat. Rev. Cancer 18, 634–645 (2018).
De Chiara, F. et al. Urea cycle dysregulation in non-alcoholic fatty liver disease. J. Hepatol. 69, 905–915 (2018).
Purwono, A. R., Hibbaan, M. & Budihardjo, M. A. Ammonia-nitrogen (NH3-N) and ammonium-nitrogen (NH4+-N) equilibrium on the process of removing nitrogen by using tubular plastic media. J. Mater. Environ. Sci. 8, 4915–4922 (2017).
Eng, C. H., Yu, K., Lucas, J., White, E. & Abraham, R. T. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci. Signal 3, ra31 (2010).
Cheong, H., Lindsten, T., Wu, J., Lu, C. & Thompson, C. B. Ammonia-induced autophagy is independent of ULK1/ULK2 kinases. Proc. Natl Acad. Sci. USA 108, 11121–11126 (2011).
Raup-Konsavage, W. M. et al. Arginase-2 mediates renal ischemia-reperfusion injury. Am. J. Physiol. 313, F522–F534 (2017).
Porcelli, V., Fiermonte, G., Longo, A. & Palmieri, F. The human gene SLC25A29, of solute carrier family 25, encodes a mitochondrial transporter of basic amino acids. J. Biol. Chem. 289, 13374–13384 (2014).
Shayakul, C., Clémençon, B. & Hediger, M. A. The urea transporter family (SLC14): physiological, pathological and structural aspects. Mol. Asp. Med. 34, 313–322 (2013).
Carlström, M. Nitric oxide signalling in kidney regulation and cardiometabolic health. Nat. Rev. Nephrol. 17, 575–590 (2021).
Bogdan, C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 36, 161–178 (2015).
Cotter, G. et al. L-NMMA (a nitric oxide synthase inhibitor) is effective in the treatment of cardiogenic shock. Circulation 101, 1358–1361 (2000).
Garvey, E. P. et al. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J. Biol. Chem. 272, 4959–4963 (1997).
Lampson, B. L. et al. Targeting eNOS in pancreatic cancer. Cancer Res. 72, 4472–4482 (2012).
Zhang, F., Liao, L., Ju, Y., Song, A. & Liu, Y. Neurochemical plasticity of nitric oxide synthase isoforms in neurogenic detrusor overactivity after spinal cord injury. Neurochem. Res. 36, 1903–1909 (2011).
Zhang, X. et al. Molecular basis for hierarchical histone de-β-hydroxybutyrylation by SIRT3. Cell Discov. 5, 35 (2019).
Huang, H. et al. The regulatory enzymes and protein substrates for the lysine β-hydroxybutyrylation pathway. Sci. Adv. 7, eabe2771 (2021).
Kaczmarska, Z. et al. Structure of p300 in complex with acyl-CoA variants. Nat. Chem. Biol. 13, 21–29 (2017).
Kurmi, K. & Haigis, M. C. Nitrogen metabolism in cancer and immunity. Trends Cell Biol. 30, 408–424 (2020).
Lee, J. S. et al. Urea cycle dysregulation generates clinically relevant genomic and biochemical signatures. Cell 174, 1559–1570 (2018).
Matsumoto, S. et al. Urea cycle disorders-update. J. Hum. Genet. 64, 833–847 (2019).
Hajaj, E., Sciacovelli, M., Frezza, C. & Erez, A. The context-specific roles of urea cycle enzymes in tumorigenesis. Mol. Cell 81, 3749–3759 (2021).
Pearce, E. L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
McGovern, N. et al. Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature 546, 662–666 (2017).
Peng, W. et al. Mitochondrial dysfunction in atherosclerosis. DNA Cell Biol. 38, 597–606 (2019).
Fouda, A. Y., Eldahshan, W., Narayanan, S. P., Caldwell, R. W. & Caldwell, R. B. Arginase pathway in acute retina and brain injury: therapeutic opportunities and unexplored avenues. Front. Pharmacol. 11, 277 (2020).
Yoo, H. C. et al. A variant of SLC1A5 Is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metab. 31, 267–283 (2020).
Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 75, 639–653 (2004).
Zhao, Y., Vanhoutte, P. M. & Leung, S. W. Vascular nitric oxide: beyond eNOS. J. Pharmacol. Sci. 129, 83–94 (2015).
Sun, J. et al. Essential role of nitric oxide in acute ischemic preconditioning: S-nitros(yl)ation versus sGC/cGMP/PKG signaling? Free Radic. Biol. Med. 54, 105–112 (2013).
Xiang, T. et al. The novel ZEB1-upregulated protein PRTG induced by Helicobacter pylori infection promotes gastric carcinogenesis through the cGMP/PKG signaling pathway. Cell Death Dis. 12, 150 (2021).
Luedike, P. et al. Cardioprotection through S-nitros(yl)ation of macrophage migration inhibitory factor. Circulation 125, 1880–1889 (2012).
Seth, D. et al. A multiplex enzymatic machinery for cellular protein s-nitrosylation. Mol. Cell 69, 451–464 (2018).
Majumdar, U. et al. Nitric oxide prevents aortic valve calcification by S-nitrosylation of USP9X to activate NOTCH signaling. Sci. Adv. 7, eabe3706 (2021).
Zhang, H. et al. TCR activation directly stimulates PYGB-dependent glycogenolysis to fuel the early recall response in CD8+ memory T cells. Mol. Cell 82, 3077–3088 (2022).
Spinelli, J. B., Kelley, L. P. & Haigis, M. C. An LC–MS approach to quantitative measurement of ammonia isotopologues. Sci. Rep. 7, 10304 (2017).
Acknowledgements
This work was supported by the Natural Science Foundation of China (81788101 to B.H. and 82071864, 82150103 to K.T.), CAMS Innovation Fund for Medical Sciences (2021-1-I2M-021 to B.H.), Program for HUST Academic Frontier Youth Team (2018QYTD01 to K.T.), the Key R&D program of Hubei Province (2020BCA068 to K.T.) and the Young Top-notch Talent Cultivation Program of Hubei Province (K.T.).
Author information
Authors and Affiliations
Contributions
B.H. conceived the project. K.T., H.Z., J.D., D.W., S.L., S.L., Q.C., C.C., J. Liu, J.C., J. Lv and J.M. conducted the experiments. K.T. and B.H. analyzed the data. B.H. and K.T. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Ping-Chih Ho and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: N. Bernard, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
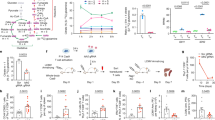
Extended Data Fig. 1 Urea cycle is active in CD8+ Tm cells.
a, Standard curve of urea cycle substances. b, The levels of hydroxyurea (CP derivant), ornithine, citrulline, argininosuccinate and arginine in CD4+ OVA specific Tn, Te and Tm cells from mice were analyzed by LC-MS/MS through standard curve. C, Te (IL-2 derived) cells treated with BPTES (10 μM) for 12 hours, then cultured with 15N-glutamine (2 mM). 24 hours later, the incorporation of 15N-glutamine into urea cycle intermediates including CP, citrulline, argininosuccinate, arginine and urea were analyzed by LC-MS/MS. In b and c, n = 3 independent experiments. Two-tailed unpaired Student’s t-tests followed by Bonferroni’s test (b and c). The data represent mean ± s.d.
Extended Data Fig. 2 Urea cycle is required for CD8+ T cell memory development.
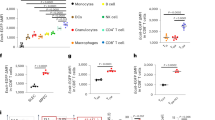
a, IL-15-derived Tm cells were treated with NH4Cl (20 mM) or erastin (10 μM) for 24 hours, lipid peroxidation was determined by flow cytometry, erastin treatment group was as a positive control. b, IL-15-derived Tm cells were treated with NH4Cl for 24 hours or TNF-α for 3 hours, the expression were analyzed by western blot, all samples were pre-treated with 20 μM Z-VAD for 30 min, TNF-α treatment group was as a positive control. c, IL-15-derived Tm cells were treated with 10 mM NH4Cl for 24 hours, the expression were detected by western blot. d, IL-2-derived Teff cells and IL-15-derived Tm cells were treated with different doses of NH4Cl for 24 hours, the expression were detected by western blot. e, The same as d, except that the LysoSensor fluorescence intensity was measured by flow cytometry. f, IL-15-derived Tm were treated with 3MA (5 mM) or NH4Cl (10 mM) for 24 hours, and then PI was analyzed. g, The knockdown efficiency of shCps1 in IL-15-derived Tm cells. h, CD8+ Teff (IL-2) and Tm (IL-15) cells were cultured with 15N-glutamine (2 mM), the incorporation of N-acetylgutamate (m + 1) were analyzed by LC-MS/MS. i, The knockdown efficiency of shGDH in IL-15-derived Tm cells. j, IL-15-derived Tm cells transduced with shGDH were cultured with 15N-glutamine (2 mM), LC-MS/MS analysis was performed for m + 1 N-acetylgutamate. k, The levels of ammonia in IL-15-derived CD8+ Tm cells transduced with shGDH were analyzed. l, The same as k, except that the cell number was analyzed. m, The gating strategy of flow cytometry analysis on Tetramer+CD45.1+CD8+ T cells. n, The same as Fig. 2k, except that on day 7, the CD45.1+Tetramer+CD8+ T cells were analyzed by flow cytometry. o, The gating strategy of flow cytometry analysis on Tetramer+ CD8+ T cells. p, CD8+ Tn (IL-7), Teff (IL-2) or Tm (IL-15) cells were cultured with 15N-glutamine (2 mM). the incorporation of NH3 (m + 1), N-acetylgutamate were analyzed by LC-MS/MS. In a-i and p, n = 3 independent experiments; n, n = 5 mice. Two-tailed unpaired Student’s t-tests(a, g-l and n),or one-way ANOVA followed by Bonferroni’s test (f and p). The data represent mean ± s.d.
Extended Data Fig. 3 CD8+ Tm cells mobilize arginase 2 for urea formation.
a, Western blot analysis of the expression of ARG1 in CD8+ Tn, Teff and Tm cells from mice or IL-2-derived Teff cells and IL-15 derived Tm cells, M2 type macrophages was as a positive control. b, The knockdown efficiency of shArg2 in IL-15-derived Tm cells. c, C57BL/6 mice were transferred with CD45.1+CD8+ OT-I T cells and infected with Lm-OVA. 7 days later, the mice were injected with nor-NOHA (10 mg/kg) or control saline once per day. On day 30, the CD45.1+CD8+ T cells in spleen were sorted, and then the ammonia and urea were detected. the level of ammonia and urea in GFP+CD8+ Tm cells from spleen were analyzed. d, The gating strategy of flow cytometry analysis on CD45.1+CD8+ T cells. e, The gating strategy of flow cytometry analysis on GFP+CD8+ T cells. f, C57BL/6 mice were transferred with GFP-shArg2 transduced CD8+ OT-I T cells, and then infected with Lm-OVA. On day 30, the GFP+CD8+ T cells in the LNs, PBMCs and spleen were analyzed by flow cytometry. In a and b,n = 3 independent experiments; c and f, n = 5 mice. Two-tailed unpaired Student’s t-tests followed by Bonferroni’s test (b and c). The data represent mean ± s.d.
Extended Data Fig. 4 SLC25A29 and SLC14A1 are utilized for urea cycle.
a, The knockdown efficiency of shSlc25a29 in IL-15-derived Tm cells. b, The knockdown efficiency of shSlc14a1 in IL-15-derived Tm cells. In a and b, n = 3 independent experiments. Two-tailed unpaired Student’s t-tests followed by Bonferroni’s test (a and b).The data represent mean ± s.d.
Extended Data Fig. 5 CD8+ Tm cells dispose ammonia by citrulline cycle.
a, The knockdown efficiency of shnNOS, shiNOS and sheNOS in IL-15-derived Tm cells. b, IL-15-derived CD8+ Tm treat with 100 μM LNMMA for 48 hours, then cultured with 15N-NH4Cl for 24 hours and LC-MS/MS analysis was performed for m + 1 citrulline, m + 1 argininosuccinate, m + 1 arginine and m + 1 urea. c, d, IL-15-derived CD8+ Tm were treated with 10 μM nor-NOHA (c) or 100 μM L-NMMA (d) for 48 hours, then were cultured with [U4] 15N-arginine for 6 hours and LC-MS/MS analysis was performed for m + 2, m + 3 citrulline, m + 2 ornithine and m + 2 urea. In a-d, n = 3 independent experiments. Two-tailed unpaired Student’s t-tests followed by Bonferroni’s test (a-d). The data represent mean ± s.d.
Extended Data Fig. 6 Cps1 is crucial for nitrogen disposal and memory development.
a, The gating strategy of flow cytometry analysis on GFP+CD8+ T cells. b,.The same as Fig. 6a, except that on day 7, CD8+GFP+ T cells were analyzed. c, The same as Fig. 6a, except that On day 30, Annexin V, Ki-67 and BrdU in spleen CD8+GFP+ Tm cells were analyzed. d, C57BL/6 mice were co-transferred with 1×104 mCherry-NC and 1×104 GFP-OE-Cps1 transduced CD8+ OT-I T cells, and then infected with Lm-OVA. On day 30, the mCherry+CD8+(NC) and GFP+CD8+(OE-Cps1) T cells in the LNs, PBMCs and spleen were analyzed by flow cytometry. e, 1×105 GFP-shNC or GFP-shCps1 CD45.1+ OT-I Tm cells were adoptively transferred into CD45.2+ mice (left), 1×105 GFP-NC or GFP-OE-Cps1 CD45.1+ OT-I Tm cells were adoptively transferred into CD45.2+ mice (right), On day 30, the CD8+CD45.1+GFP+ T cells in the spleen were analyzed by flow cytometry. f, The gating strategy of flow cytometry analysis on CD45.1+CD8+ T cells. g, Mice were transferred with 1×105 OT-l T cells transduced with shCps1, 30 days later; the percentage of cytokine-producing CD8+ OT-l cells were analyzed 4 h after Lm-OVA reinfection. h, The same as g, except that mice were transferred with 1×105 OT-l T cells transduced with OE-Cps1. i, 1×105 GFP-shNC or GFP-shCps1 CD45.1+ OT-I Tm cells were adoptively transferred into naïve mice followed by infection with Lm-OVA, 5 days after infection, the percentage and cell numbers of CD8+ OT-I T cells are analyzed. j, The same as i, except that 1×105 NC or OE-Cps1 CD45.1+ OT-I Tm cells were adoptively transferred into naive mice followed by infection with Lm-OVA. k, 1×105 shNC or shCps1 CD45.1+ OT-I Tm cells were adoptively transferred into naïve mice(left), or 1×105 NC or OE-Cps1 CD45.1+ OT-I Tm cells were adoptively transferred into naive C57BL/6 mice (right), followed by infection with Lm-OVA, 5 days after infection, the percentage of cytokine-producing CD8+ OT-I T cells were analyzed. In a-k, n = 5 mice. Two-tailed unpaired Student’s t-tests followed by Bonferroni’s test (b,e and g-k). The data represent mean ± s.d.
Extended Data Fig. 7 β-hydroxybutyrylation of Cps1 triggers CP metabolic cascade.
a, The knockdown efficiency of shBdh1 in IL-15-derived Tm cells. b, IL-15-derived Tm cells transduced with shBdh1, the expression of Cps1 was analyzed by qPCR. In a and b, n = 3 independent experiments. Two-tailed unpaired Student’s t-tests followed by Bonferroni’s test (a and b). The data represent mean ± s.d.
Supplementary information
Supplementary Tables 1–4
Reagent information, sequences of shRNA information, antibody information and sequences of qPCR primer information.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 2
Unprocessed immunoblot.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 3
Unprocessed immunoblot and unprocessed fluorescent image.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 4
Unprocessed immunoblot and unprocessed fluorescent image.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 5
Unprocessed immunoblot.
Source Data Fig. 6
Statistical Source Data.
Source Data Fig. 7
Statistical Source Data.
Source Data Fig. 7
Unprocessed immunoblot.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 2
Unprocessed immunoblot.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 3
Unprocessed immunoblot.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 7
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, K., Zhang, H., Deng, J. et al. Ammonia detoxification promotes CD8+ T cell memory development by urea and citrulline cycles. Nat Immunol 24, 162–173 (2023). https://doi.org/10.1038/s41590-022-01365-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-022-01365-1
This article is cited by
-
Cellular metabolism regulates the differentiation and function of T-cell subsets
Cellular & Molecular Immunology (2024)
-
eUREkA! T cells answer nature’s call
Cellular & Molecular Immunology (2023)
-
The malate shuttle detoxifies ammonia in exhausted T cells by producing 2-ketoglutarate
Nature Immunology (2023)
-
Research progress on the role of cationic amino acid transporter (CAT) family members in malignant tumors and immune microenvironment
Amino Acids (2023)