Abstract
Understanding immune responses to SARS-CoV-2 messenger RNA (mRNA) vaccines is of great interest, principally because of the poor knowledge about the mechanisms of protection. In the present study, we analyzed longitudinally B cell and T cell memory programs against the spike (S) protein derived from ancestral SARS-CoV-2 (Wuhan-1), B.1.351 (beta), B.1.617.2 (delta) and B.1.1.529 (omicron) variants of concern (VOCs) after immunization with an mRNA-based vaccine (Pfizer). According to the magnitude of humoral responses 3 months after the first dose, we identified high and low responders. Opposite to low responders, high responders were characterized by enhanced antibody-neutralizing activity, increased frequency of central memory T cells and durable S-specific CD8+ T cell responses. Reduced binding antibodies titers combined with long-term specific memory T cells that had distinct polyreactive properties were found associated with subsequent breakthrough with VOCs in low responders. These results have important implications for the design of new vaccines and new strategies for booster follow-up.
Similar content being viewed by others
Main
In the last 2 years, infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1 has caused remarkable morbidity and mortality. There are hopes that herd immunity, achieved through both natural SARS-CoV-2 infection and vaccination, can control it2. Memory B cells, CD4+ T cells and CD8+ T cells elicited by SARS-CoV-2 mRNA vaccination have a critical role in the protection against infection and represent key determinants in the vaccine boost2. Both Pfizer/BioNTech and Moderna mRNA vaccines show 91% and 93% protective immunity over 7 months3,4,5, respectively, and up to 95% efficacy in preventing symptomatic COVID-19 disease caused by the wild-type (WT) SARS-CoV-2 and delta and omicron VOCs6,7,8.
Analyses of SARS-CoV-2-infected individuals has indicated the activation of both B and T cells in some subjects, but also discordant results in others9. Although studies in Middle East respiratory syndrome and SARS-CoV-1 infections suggest the long persistence of T cells and their ability to confer protection10,11, the extent to which vaccine-elicited CD4+ and CD8+ T cell immunity is a correlate of protection against SARS-CoV-2 and VOCs remains poorly understood. The contribution of T cell immunity may in fact be superior compared with antibody protection against divergent VOCs, such as omicron, which escape neutralizing antibodies, as reported in nonhuman primates12. Based on these considerations, understanding the mechanisms of protective B and T cell memory responses would allow the development of new vaccination protocols and follow-ups to monitor protection.
In the case of subjects infected with SARS-CoV-2, natural immunity has been reported to be 93–100% protective against symptomatic disease for at least 8 months13,14,15. As such, individuals resolving previous SARS-CoV-2 infection have been vaccinated within 12 months after infection to achieve long-term protection (hybrid immunity)16,17,18. Hybrid immunity elicits substantially higher amounts of cross-variant neutralizing antibodies compared with those elicited by the vaccination of naive donors16,17,18,19. This strong immunity may be linked to the higher clonal turnover of B cells and greater somatic hypermutation in SARS-CoV-2-recovered compared with naive vaccinated individuals20,21. Although several studies have reported the contribution of virus-specific B and T cells to the protective immunity or immunopathology in SARS-CoV-2-vaccinated, -recovered or -infected individuals18,22,23,24, it is still unclear whether hybrid immunity generates a more robust repertoire of memory B and T cells when compared with immunity generated after vaccination.
The emergence of the new VOCs such as B.1.351 (beta), B.1.617.2 (delta) and B.1.1.529 (omicron) has brought some insight into the mechanisms of protection of the mRNA vaccines against these mutants and the quality and persistence of cross-reactive memory T cells2,25,26,27,28. However, although long-term memory B cell and CD4+ T cell responses to vaccination against SARS-CoV-2 start to be elucidated8,29,30, the diversification of CD4+ and CD8+ T cells is not completely understood. This is particularly relevant for delta and omicron, which can evade antibody responses and have been correlated with lower vaccine efficacy against COVID-19 symptoms31,32, although only a minimal number of spike-specific major histocompatibility complex I epitopes are muted compared with WT S (SWT)33.
In the present study, we analyzed the S-specific antibody durability in correlation with memory B cells, CD4+ T cells and CD8+ T cells in SARS-CoV-2-vaccinated naive (VN) and SARS-CoV-2-vaccinated COVID-19-recovered (VR) individuals. Based on B cell responses and distinct polyreactivity of memory CD4+ and CD8+ T cells, VN and VR individuals could be classified as high responders (HRs) and low responders (LRs). Responses in VNHRs were mainly characterized by increased frequencies of S-specific CCR7+CD27+CD45RA-CD4+ central memory 1 T (TCM1) cells and CCR7+CD27+CD45RA-CD8+ TCM1 cells when compared with VNLRs. Responses in VNHRs resembled those of VR individuals, whereas VNLRs were characterized by the accumulation of effector memory 2 T (TEM2) cells. These distinct immune responses correlated with protection against COVID-19 caused by infection with delta and omicron in VNHRs and VRHRs.
Results
Distinct SWT antibody titers in VN and VR subjects after vaccination
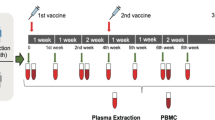
We longitudinally examined a cohort of 379 healthcare workers (69% females, Candiolo Cancer Institute (CCI), a certified COVID-free hospital) who were vaccinated with three doses of the BNT162b2 mRNA vaccine encoding the SWT (ref. 7) at time 0 (T0, first dose), week 3 (second dose) and month 9 after the first dose (third dose) (Fig. 1a and Supplementary Table 1). Based on the results of previous SARS-CoV-2 mRNA PCR tests, COVID-19 diagnosis and antibody titers against the SWT and nucleocapsid (NWT) subjects were classified as: SARS-CoV-2 naive (N), no history of infection, negative mRNA PCR swabs throughout all tests (for testing protocols, see Methods) and negative antibody responses to SWT and NWT (n = 307, 67% females); seropositive, no history of infection based on negative mRNA PCR tests, but low titers of antibodies specific to SWT and/or NWT (n = 24, 79% females); and recovered (R), with a past infection event documented by positive SARS-CoV-2 mRNA PCR swabs and mild COVID-19 symptoms (n = 48, 70% females) (Fig. 1b,c and Extended Data Fig. 1a,b). Peripheral blood was collected a few hours before the administration of the first vaccine dose (T0), and week 3, week 6, month 3, month 6 and month 10 postvaccination.
a, Experimental design of the study in which 379 individuals were vaccinated with the BNT162b2 mRNA vaccine and PBMCs were isolated before the administration of the first vaccine dose (T0), before the administration of the second vaccine dose at week 3 (3W), week 6 (6W), month 3 (3M) and month 6 (6M) and 1 month after the administration of the third vaccine dose, corresponding to month 10 (10M) after the first vaccine dose, followed by the analysis of humoral and T cell responses against the SARS-CoV-2 spike (S) proteins corresponding to the wild type (SWT), beta (SB), delta (SD) and omicron (SO) variants. b, Time series plots of SWT IgG titers measured in SARS-CoV-2-naive, seropositive and SARS-CoV-2-recovered subjects. Abs, antibodies; a.u., arbitrary units; F, female; M, male, NR, nonresponder. Upper LOD = 160 a.u. ml−1. c, ELISA of NWT antibody titers in naive (n = 307) and recovered (n = 48) subjects measured before vaccination (T0). All data are shown as ELISA endpoint titers (ETs) based on a standard. The dotted line indicates the LOD. d, Kernel density estimation for SWT IgG titer distribution at 3M after two vaccine doses in vaccinated naive high and low responders (VNHRs and VNLRs; left) and in vaccinated recovered high and low responders (VRHRs and VRLRs; right). The curves are composed of 32,768 points with a distance between adjacent points of 5.82 × 10−3 a.u. ml−1. The total area under the density curve (A = 1) is estimated through the Riemann sum with a normalizing constant of 0.999801. e, Box plots of SWT IgG titers in VNLRs and VNHRs at T0, 3W, 6W, 3M and 6M. f, Pie charts of VNLRs, VNHRs, VRLRs and VRHRs at month 3 postvaccination, with annotations for number and percentage of females and males. Statistics were calculated using two-sided Wilcoxon’s rank-sum test (c and e) or signed-rank test (b), with Benjamini–Hochberg correction for multiple testing. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. The boxes show median, upper and lower quartiles and the whiskers show 1.5× the interquartile range (IQR) on either side (b and e) or minimum/maximum (c). Each dot represents one subject and the data are pooled.
Based on the SWT-specific immunoglobulin (Ig)M and IgG titers in plasma during the first 6 months postvaccination, 10 (of the 379, 67% females) participants did not respond to the vaccine (Fig. 1b and Extended Data Fig. 1b). At each time point, the median of SWT-specific IgM titers was not significantly different between VN and VR subjects (Extended Data Fig. 1b). In contrast, VR subjects had higher baseline and a delayed decrease in SWT-specific IgG concentration compared with VN subjects at all timepoints (Fig. 1b). In particular, SWT-specific IgG amounts significantly increased compared with T0 at weeks 3 and 6 postvaccination and gradually decreased at months 3 and 6 in VN subjects, whereas they remained high at month 3 and decreased at month 6 in VR subjects, although less dramatically than in VN subjects (Fig. 1b). After a peak between weeks 1 and 3, the SWT-specific IgG titers declined between months 3 and 6 in VN subjects (Fig. 1b). At month 3 postvaccination, the Kernel density estimation of SWT-specific IgG titers showed a bimodal distribution in VN subjects (Fig. 1d). Based on the antimode value (that is, the least frequent value between the modes, cut-off 135 a.u. ml−1), the 298 VN subjects were divided into VNLRs (n = 236, 64% females) and VNHRs (n = 62, 81% females) (Fig. 1e). Similarly, based on the antimode value (112 a.u. ml−1), the VR cohort was classified as VRHRs (n = 39, 72% females) and VRLRs (n = 9, 56% females) (Fig. 1d and Extended Data Fig. 1c). Both VNHRs and VNLRs were negative for NWT-specific IgGs (Fig. 1c). Of note, at month 3 postvaccination, there was increased frequency of females among VNHRs (81%) compared with LRs (64%, Fisher’s test P = 0.01481; Fig. 1f). No correlation was found between age and SWT-specific IgG titers at month 6 postvaccination (Extended Data Fig. 1d), although SWT-specific IgG titers were significantly higher in the 20- to 29-year-old group than in all the others (Extended Data Fig. 1e). These results indicated durable SWT-specific IgG responses in all cohorts, with higher titers in VR than VN subjects, and distinguished VNHRs and VNLRs based on the bimodal distribution of SWT-specific IgG titers.
mRNA vaccine induces B cell responses and RBD antibodies
To determine whether the SWT-specific IgG titers correlated with B cell frequencies, peripheral blood mononuclear cells (PBMCs) collected at T0, week 6 and month 6 postvaccination were stained with SARS-CoV-2 SWT tetramers in the presence of antibodies for CD19, CD27, IgG, IgA and IgM (Fig. 2a and Extended Data Fig. 2a). No difference emerged in terms of the percentage of CD19+ B cells at all timepoints tested (Extended Data Fig. 2b). Lower percentages of SWT-specific B cells were found in VNLRs and VNHRs compared with VR subjects at T0 and week 6 postvaccination (Fig. 2b and Extended Data Fig. 2a,c), but the frequency of SWT-specific B cells increased significantly in VNHRs compared with VNLRs at month 6 postvaccination (Fig. 2b). The SWT-specific IgG titers correlated with the frequency of SWT-specific tetramer+ B cells at month 6 in VNHRs and VR subjects (Fig. 2c). The frequency of SWT-specific tetramer+ B cells and CD27+ IgG+ B cells were higher in VR subjects than in both VNLRs and VNHRs, especially at week 6 postvaccination (Fig. 2b,d). However, at month 6, the frequencies of SWT-specific B cells and CD27+ IgG+ B cells equalized in VR subjects and VNHRs, whereas the frequency of SWT-specific B cells and the numbers of CD27+IgG+ B cells remained significantly lower in VNLRs than in the other two cohorts (Fig. 2b,d). The frequencies of SWT-specific CD27+IgM+ B cells decreased in VNHRs and VNLRs (Extended Data Fig. 2d), whereas the frequency and numbers of SWT-specific CD27+IgA+ B cells increased (Fig. 2e and Extended Data Fig. 2e) at month 6 postvaccination.
a, Representative flow cytometry dot plots of SWT-tetramer+ cells on gated CD19+ B cells and of CD27+ IgG+ cells on gated SWT-tetramer+ B cells from VNLR, VNHR and VR subjects at month 6. b, Flow cytometry analysis showing the percentage of SWT-tetramer+ B cells in VNLR, VNHR and VR subjects at T0, week 6 and month 6. c, Spearman’s correlation plot between SWT IgG titers and percentage of SWT-tetramer+ B cells in VNLR, VNHR and VR subjects at month 6 measured by flow cytometry; VNLRs: R = 0.388 and P = NS (not significant); VNHRs: R = 0.4471 and P < 0.05; VR subjects: R = 0.4384 and P < 0.05. d,e, Flow cytometry analysis showing the percentage and number per 106 PBMCs of CD27+IgG+SWT+ B cells (d) and the percentage of CD27+IgA+SWT+ B cells (e) in VNLR, VNHR and VR subjects at T0, week 6 and month 6. Below: percentages of subjects with active responses > 0.01. The dashed black line shows the LOD. The same subjects were analyzed longitudinally at T0: VNLRs (n = 25), VNHRs (n = 24), VR subjects (n = 12); 6 W: VNLRs (n = 12), VNHRs (n = 17), VR subjects (n = 10); and 6 M: VNLRs (n = 26), VNHRs (n = 29), VNR subjects (n = 22). The box plots indicate median, IQR and minimum/maximum. Each dot of the box plots represents the average across three technical replicates of the same subject; the data are pooled from multiple experiments. Statistics were calculated using two-sided Wilcoxon’s rank-sum test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Next we measured the antibody titers against SWT receptor-binding domain (RBDWT) in the plasma collected from 30 VNHRs, 30 VNLRs and 26 VR subjects at T0, month 3 and month 6 (Fig. 3a and Extended Data Fig. 3a). Although RBDWT-specific antibodies were not detected in VNHRs and VNLRs at T0, robust RBDWT-specific antibody titers were detected in VNHRs and VR subjects compared with VNLRs at month 6 postvaccination (Fig. 3a). A similar range of percentage values was detected for delta RBD (RBDD) IgGs, whereas omicron RBD (RBDO) crossbinding IgG titers were reduced in both VNHRs and VR subjects at months 3 and 6 (Fig. 3a). The RBDD and RBDO antibody titers were always lower in VNLRs than in the other 2 cohorts at months 3 and 6 postvaccination (Fig. 3a).
a, ELISA showing SARS-CoV-2 S-RBDWT, -RBDD and -RBDO IgG titers in VNLRs, VNHRs and VR subjects in plasma at T0, 3M and 6M. ELISA results shown as ETs based on a standard. The dotted lines represent the LOD. At T0: VNLRs (n = 30), VNHRs (n = 30), VR subjects (n = 26); 3M: VNLRs (n = 28), VNHRs (n = 27), VR subjects (n = 25); 6M: VNLRs (n = 30), VNHRs (n = 29), VR subjects (n = 26). b, Neutralization assay showing 50%neutralizing dose titers (ND50) against S-RBDWT, -RBDD and -RBDO measured by pseudovirus (PSV)-neutralizing assay in plasma from VNLRs (n = 20) and VNHRs (n = 20) at month 6. c, Spearman’s correlation plots showing PSV ND50 and IgG ELISA ETs against S-RBDWT, -RBDD and -RBDO in plasma from VNLRs (n = 20) and VNHRs (n = 20) at month 6. VNLRs, RBDWT: R = 0.721 and P < 0.001, RBDD: R = 0.2305 and P = NS, RBDO: P = NS; VNHRs, RBDWT: R = 0.2168 and P = NS, RBDD: R = 0.6282 and P < 0.01, RBDO: R = 0.4277 and P < 0.01. NA indicates no neutralizing activity. The same subjects were longitudinally analyzed. The box plots indicate median, IQR and minimum/maximum. Each dot of box plots represents the average across two (a) and three (b) technical replicates of the same subject; the data are pooled from multiple experiments. Statistics were calculated using two-sided Wilcoxon’s signed-rank and two-sided Wilcoxon’s rank-sum test. **P < 0.01; ***P < 0.001; ****P < 0.0001.
To quantify the corresponding neutralizing capacity of S RBD-specific antibodies, we generated pseudotype viruses encoding S-RBDWT, -RBDD and -RBDO. At month 6, VNLRs had reduced ancestral pseudovirus-neutralizing responses compared with VNHRs, with minimal activity against delta and complete absence of neutralization against omicron particles (Fig. 3b), and the reduced neutralization against the ancestral pseudoviruses in VNLRs correlated with the reduced RBDWT antibody titers (Fig. 3c). Overall, these results indicated poor crossbinding of RBD-specific antibodies in VNLRs against delta and omicron variants, with almost complete lack of neutralizing activity 6 months postvaccination.
T cell polyreactivity varies among VNLRs, VNHRs and VR subjects
To determine the polyreactivity of memory T cell responses against SWT, SD, SO and beta S (SB), PBMCs were stimulated for 24 h with peptide megapools spanning the aforementioned proteins, whereas PBMC cultures in medium or with interleukin (IL)-2 and CD3 plus CD28 antibodies were used as negative (background) or positive controls, respectively. To measure the CD4+ and CD8+ T cell responses elicited by vaccination, we quantified the frequency of CD25+CD69+ T cells in the PBMCs from 25 VNLRs, 24 VNHRs and 12 VR subjects 24 h poststimulation and after background subtraction (as the percentage of CD25+CD69+ T cells measured in the paired PBMCs cultured in medium) at T0 and month 6 postvaccination (Fig. 4a and Extended Data Fig. 4a–c). No difference was observed in terms of percentages of total CD4+ and CD8+ T cells before stimulation (Extended Data Fig. 4d,e) and background activation (Extended Data Fig. 4f,g) in all three cohorts. The percentages of polyspecific CD4+ T cells to both SWT and SB peptide megapools were significantly higher in VR subjects than in VNHRs and VNLRs at T0 (Fig. 4b and Extended Data Fig. 4f). The percentage of CD25+CD69+CD4+ T cells significantly increased on stimulation with S-peptide megapools in both VNHRs and VNLRs at month 6 compared with T0 (Fig. 4b and Extended Data Fig. 5a). Similar observations were made for CD25+CD69+CD8+ T cell responses to the SWT, SD and SO (Fig. 4c and Extended Data Figs. 4g and 5b). No differences were observed in terms of activation with the positive controls in all examined conditions (Extended Data Fig. 5c,d). Stimulation with SWT, SD and SO peptide megapools elicited a consistent increase in the percentage of CD25+CD69+CD8+ T cells in both VNHRs and VNLRs at month 6 postvaccination (Fig. 4c and Extended Data Fig. 5b).
a, Representative flow cytometry dot plots of CD25 versus (vs) CD69 cells on gated CD4+ (left) and CD8+ (right) T cells from VNLRs, VNHRs and VR subjects at month 6, after 24h incubation with medium (Unstimulated) or SWT peptide megapools. b,c, Flow cytometry analysis showing the percentage of CD25+CD69+ cells in CD4+ T cells (b) and CD8+ T cells (c) from VNLRs, VNHRs and VR subjects at T0 and 6M, 24h after incubation with SWT, SD, SO or SB. Below: percentages of subjects with active responses >0.01. d, Flow cytometry analysis showing the percentage of IFN-γ+ cells in CD4+ T cells and CD8+ T cells from VNLRs, VNHRs and VR subjects at month 6, after 14h incubation with SWT, SD, SO or SB. Below: percentages of subjects with active responses >0.01. Data are represented with background subtraction from paired unstimulated controls. The same subjects were analyzed longitudinally at T0 and 6M: VNLRs (n = 25), VNHRs (n = 24) and VR subjects (n = 12). The box plots indicate median, IQR and minimum/maximum. Each dot of the box plots represents the average across three technical replicates of the same subject; the data are pooled from multiple experiments. Statistics were calculated using two-sided Wilcoxon’s signed-rank and two-sided Wilcoxon’s rank-sum test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
We next assessed the effector T cell activity by investigating the expression of granzyme B and the effector cytokines interferon (IFN)-γ, tumor necrosis factor (TNF)-α and IL-2 after 14 h of stimulation of PBMCs isolated at T0 and month 6 with S-peptide megapools derived from WT, delta, omicron and beta variants, using PBMCs cultured with IL-2 and CD3 plus CD28 antibodies or medium as positive or negative controls, respectively (Extended Data Fig. 6a). There were no differences in the percentage of granzyme B+ cells between T0 and month 6 in both VNLR and VNHR subjects (data not shown), whereas IFN-γ expression was elicited by all S-derived megapools in antigen-specific CD4+ T cells and CD8+ T cells, although at different levels, at month 6 (Fig. 4d). A significant increase in the T cell responses to SO peptides was observed in VNHRs and VR subjects compared with VNLRs (Fig. 4d), indicating a higher frequency of polyreactive T cells in those samples. By contrast, the percentages of IL-2+ (Extended Data Fig. 6b) and TNF-α+CD4+ or -CD8+ T cells (Extended Data Fig. 6c) were quite similar across all cohorts, as well as in the PBMCs stimulated with IL-2 and CD3 plus CD28 antibodies (positive controls; Extended Data Fig. 6d). These results showed that polyreactive, S-specific memory CD4+ and CD8+ T cell responses could be detected until month 6 postvaccination and that the frequencies of IFN-γ+CD4+ T cells or IFN-γ+CD8+ T cells reactive against SO were reduced in VNLRs compared with VNHRs and VR individuals.
Distinct memory T cell subsets react against SWT and So
Several subsets of CD4+ and CD8+ T cells, such as CCR7+CD45RA− TCM cells, CCR7−CD27+CD45RA- TEM1 cells, CCR7−CD27-CD45RA- TEM2 cells and terminally differentiated effector memory CD45RA+ T cells (TEMRA cells) have been described34,35,36,37. Based on immune phenotype, transcriptional and epigenetic programs, CD8+ TEM1 cells are closely related to CD8+ TCM cells, and have been suggested to exert memory functions in peripheral tissues34, whereas CD8+ TEM2 cells resemble differentiated CD8+ TEMRA cells34,37. The role of these T cell subsets in protection against infections is still unclear. To evaluate the heterogeneity of memory CD4+ and CD8+ T cells, PBMCs collected at T0 and month 6 from 25 VNLRs, 24 VNHRs and 12 VR subjects were stimulated for 24 h with peptides covering SWT, SD, SB and SO, or with positive (IL-2 and CD3 plus CD28 antibodies) and negative (medium) controls. The expression of several differentiation markers was assessed on gated S-specific CD25+CD69+CD4+ T cells and CD25+CD69+CD8+ T cells by flow cytometry to distinguish naive CD45RA+CCR7+CD27+ T cells (TN cells), CCR7+CD27+CD45RA− TCM1 cells, CCR7+CD27−CD45RA− TCM2 cells, CCR7−CD27+CD45RA− TEM1 cells, CCR7−CD27−CD45RA− TEM2 cells and CCR7−CD45RA+TEMRA cells (Fig. 5a, Extended Data Figs. 4b,c, 7a–f and 8a–f). VR subjects had the highest frequencies of CD4+ TCM1 cells and CD4+ TEM1 cells in response to all S megapools at T0 (Fig. 5a–c and Extended Data Fig. 7a,b). Some VN subjects (<20% of responders in all conditions) showed cross-reactive responses to S peptides of CD4+ TCM1 cells at T0 (Fig. 5b), probably as a result of T cell responses elicited after SARS-CoV-2 exposure without infection or infections with α- and β-coronaviruses29,38,39,40. The percentages of CD25+CD69+CD4+ TCM1 cells specific for SD, SO and SB were significantly higher in VNLRs at month 6 compared with T0, as well as in VNHRs activated with all S-peptide megapools, except for SB (Extended Data Fig. 7a). However, the frequency of SWT- and SD-specific CD4+ TCM1 cells was significantly reduced in VNLRs compared with the frequency of S-specific CD4+ TCM1 cells in VNHRs and VR subjects (Fig. 5b). The frequencies of SWT-specific CD4+ TEM1 cells were increased in all cohorts at month 6 compared with T0 (Extended Data Fig. 7b), but increased frequencies of polyreactive CD4+ TEM1 cells were measured only in VNHRs (Extended Data Fig. 7b) at month 6 postvaccination. Stronger cross-reactive CD4+ TEM1 cells were also measured in VR subjects compared with VNHRs and VNLRs at month 6 postvaccination (Fig. 5c). Last, the percentage of SWT- and SD-specific CD4+ TEM2 cells was significantly increased in VNLRs after vaccination (Extended Data Fig. 7c), and they significantly increased in VNHRs and VNLRs compared with VR subjects (Fig. 5d). No differences were observed when the PBMCs were stimulated with a positive control (Extended Data Fig. 8a,c,e). Higher frequencies of S-specific CD4+ TN cells were measured in VNHRs compared with VNLRs before vaccination (T0; Extended Data Fig. 9a), suggesting a wider T cell receptor (TCR) repertoire in the TN cell subset in VNHRs. We did not detect differences in the percentage of CD4+ TCM2 and CD4+ TEMRA subsets in all cohorts and timepoints (Extended Data Fig. 9b,c).
a, Representative flow cytometry dot plots of CCR7 and CD27 on gated CD25+CD69+CD45RA-CD4+ T cells in VNLRs, VNHRs and VR subjects at month 6, after 24h incubation with medium (unstimulated) or SWT. b–d, Flow cytometry analysis showing the percentage of CCR7+CD27+ CD45RA- TCM1 (b), CCR7−CD27+CD45RA- TEM1 (c) and CCR7−CD27−CD45RA- TEM2 (d) cells on gated CD25+CD69+CD4+ T cells collected at T0 and 6M, after 24h incubation with SWT, SD, SO or SB. Below: percentages of subjects with TCM1 (b), TEM1 (c) and TEM2 (d) CD25+CD69+CDR45RA- CD4+ T cell active responses >LOS. Data are represented with background subtraction from paired unstimulated controls. The dotted black lines represent the LOD and the dotted green lines the LOS. The same subjects were longitudinally analyzed: VNLRs (n = 25), VNHRs (n = 24) and VR subjects (n = 12). The box plots indicate median, IQR and minimum/maximum. Each dot of box plots represents the average across three technical replicates of the same subject; the data are pooled from multiple experiments. Statistics were calculated using two-sided Wilcoxon’s signed-rank and two-sided Wilcoxon’s rank-sum test. *P < 0.05; **P < 0.01.
Vaccination promoted a significant increase in the magnitude of percentages of S-specific CD8+ TCM1 cells in VNHRs (Extended Data Fig. 7d), but only SWT- and SB-specific CD8+ TCM1 cells in VNLRs (Extended Data Fig. 7d) at month 6 postvaccination compared with T0. VR subjects also had S-specific CD8+ TCM1 cell responses at month 6 (Fig. 6a,b and Extended Data Fig. 7d). Of note, there was a significant reduction in the response of VNLR CD8+ TCM1 cells to SWT, SO and SD peptide megapools compared with VNHRs (Fig. 6b). We did not observe major differences in the reactivity of CD8+ TEM1 cells of VNHRs compared with VNLRs before and after vaccination (Fig. 6a,c and Extended Data Fig. 7e), whereas a significant enrichment of S-polyreactive CD8+ TEM2 cells was measured in VNLRs at month 6 postvaccination compared with T0 (Extended Data Fig. 7f). Similar frequencies of CD8+ TN cells were found at T0 and month 6 (Extended Data Fig. 9d), whereas CD8+ TCM2- and CD8+ TEMRA-specific responses, in terms of percentages, were barely detectable at both timepoints (Extended Data Fig. 9e,f). These observations indicated that, at month 6 postvaccination, higher frequencies of polyreactive CD4+ TCM1 cells (Fig. 5b) and CD8+ TCM1 cells (Fig. 6b) were present in VNHRs compared with VNLRs, whereas VNLR memory responses were characterized by the accumulation of CD4+ (Fig. 5d and Extended Data Fig. 7c) and CD8+ (Fig. 6d and Extended Data Fig. 7f) TEM2 cells.
a, Representative flow cytometry dot plots of CCR7 and CD27 on gated CD25+CD45RA-CD69+ CD8+ T cells in VNLRs, VNHRs and VR subjects at month 6, measured by flow cytometry, after 24h incubation with medium (unstimulated) or SWT. b–d, Flow cytometry analysis showing the percentage of CCR7+CD27+CD45RA- TCM1 (b), CCR7-CD27+CD45RA- TEM1 (c) and CCR7-CD27-CD45RA- TEM2 (d) cells among CD25+CD69+CD8+ T cells collected at T0 and 6M, after 24h incubation with SWT or SD, SO and SB. Below: percentage of subjects with TCM1 (b), TEM1 (c) and TEM2 (d) CD25+CD69+CD45RA-CD8+ T cell active responses >LOS. Data are represented with background subtraction from paired unstimulated controls. The dotted black lines represent the LOD and the dotted green lines the LOS. The same subjects were longitudinally analyzed: VNLRs (n = 25), VNHRs (n = 24) and VR subjects (n = 12). The box plots indicate median, IQR and minimum/maximum. Each dot of box plots represents the average across three technical replicates of the same subject; the data are pooled from multiple experiments. Statistics were calculated using two-sided Wilcoxon’s signed-rank test and two-sided Wilcoxon’s rank-sum test. *P < 0.05; **P < 0.01; ****P < 0.0001.
RBD antibodies and T cells correlate with COVID-19 protection
To investigate how VOC-specific immune responses are modulated after the third vaccine dose and how they correlate with protection, we examined the VOC–RBD-binding antibodies and the T cell responses against SWT, SD, SO and SB at 1 month post-BNT162b2 mRNA vaccine booster, which was administered to all participants at month 9 after the first dose. The RBDWT- and RBDD -binding antibody titers were reduced in VNLRs compared with VNHRs and VR subjects even at month 10 (corresponding to 1 month after the third booster) (Fig. 7a, upper panel). However, the postbooster at month 10 RBD-specific antibody titers were significantly increased in all cohorts, 10-fold for RBDWT and RBDD and 100-fold for RBDO compared with month 6 (Fig. 7a, lower panel). The fold-change for RBDO was significantly lower in VNLRs and VR subjects than in VNHRs (Fig. 7a, upper panel). At month 1 postbooster, activated CD25+CD69+CD4+ T cells were detected in both VNHRs and VNLRs after stimulation with S (Fig. 7b); however, there was no significant increase in the frequencies of S-specific CD25+CD69+CD4+ T cells postboost compared with month 6 postvaccination, except for the SWT-specific CD25+CD69+CD4+ T cells in VNHRs (Fig. 7c and Extended Data Fig. 10a). The frequencies of CD25+CD69+CD8+ T cells in VNLRs were low at month 1 postboost (Fig. 7d and Extended Data Fig. 10b), without any significant increase compared with month 6 postvaccination (Fig. 7e).
a, ELISA showing S-RBDWT, -RBDD and -RBDO binding IgG titers in VNLRs, VNHRs and VR subjects at month 10 (upper panel) and the corresponding ratio vs pre-boost values at month 6 (lower panel). ELISA results shown as ETs based on a standard. The dotted lines represent the LOD. At month 10: VNLRs (n = 24), VNHRs (n = 20) and VR subjects (n = 17). At month 10 vs 6: VNLRs (n = 23), VNHRs (n = 19) and VR subjects (n = 13). b–e, Flow cytometry analysis showing the percentage of CD25+CD69+ cells on gated CD4+ (b) and CD8+ (d) T cells from VNLRs (n = 13) and VNHRs (n = 13) at month 10, after 24h incubation with SWT, SD, SO or SB, and the corresponding ratio vs pre-boost values at month 6 (c and e). ND, not detected. Below: percentages of subjects with active responses >0.01. Data are represented with background subtraction from paired unstimulated controls. The same subjects were longitudinally analyzed. The box plots indicate the median, IQR and minimum/maximum. Each dot of the box plots represents the average across two (a) and three (b–e) technical replicates of the same subject. Statistics were calculated using two-sided Wilcoxon’s signed-rank test and two-sided Wilcoxon’s rank-sum test. The significance of the ratio was assessed by Wilcoxon’s signed-rank t-test compared with a hypothetical median of 1. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Last, to test whether the different humoral responses and TM cells’ fate diversification correlated with protection, we assessed how many vaccinated individuals had breakthrough infections with symptoms after immunization with two or three vaccine doses. The SARS-CoV-2 pandemic in Italy has been characterized by four distinct infection waves (www.ecdc.europa.eu), with delta accounting for >90% of the virus sequences in December 2021, and omicron for >90% since the beginning of 2022 (Fig. 8a). Of the study subjects, 1.5% (5/346) were infected with SARS-CoV-2 and developed mild COVID-19 (assessed by positive SARS-CoV-2 PCR swabs, cough and a few days of fever) within 8 months postvaccination, corresponding to the delta wave (Fig. 8b), and 7.33% (25/341) were infected with SARS-CoV-2 between 10 and 15 months postvaccination, corresponding to omicron (Fig. 8a). All subjects who developed COVID-19 symptoms after the second vaccine dose were VNLRs (5 subjects, 2.12%), (Fig. 8b). Among the VNLR subjects, 9.1% (21/231) developed symptomatic disease after three vaccine doses, corresponding to the omicron wave (Fig. 8b), suggesting that the reduced humoral and cellular protection in the VNLR cohort correlated with reduced protection (Fisher’s exact test, P = 0.01). Two VNHRs developed asymptomatic breakthrough infections during the omicron wave (Supplementary Table 2). Among the VR subjects, 4 of the 9 VRLRs (44.4%) developed COVID-19 (Fisher’s exact test, P = 0.0006) (Supplementary Table 2) and none of the VRHRs (Fig. 8b). These results indicated that the reduced VOC–RBD-binding antibody titers, combined with the reduced S-specific T cell responses, correlated with the increased susceptibility of VNLRs to break through COVID-19 caused by omicron (Supplementary Table 3).
a, Prevalence of the indicated variants from January 2021 to 15 April 2022 in Italy. b, Percentage of COVID-19 cases correlated with the indicated SARS-CoV-2 variant breakthrough in VNLRs, VNHRs, VRLRs and VRHRs after two (left) and three (right) vaccine doses. Fisher’s exact test was calculated in J (P = 0.01 VNLRs versus VNHRs; P = 0.0006 VRLRs versus VRHRs).
Discussion
In the present study we show that BNT162b2 mRNA vaccine elicits broadly long-term polyreactive humoral and cellular immunity against S derived from the most widespread VOCs, including omicron. Based on the bimodal distribution of the SWT IgG antibody titers at month 3 postvaccination, we subdivided the VN subjects into VNHRs, who showed long-lasting and higher titers of S- and RBD-specific IgGs, similar to those observed in VR subjects, which are characterized by robust antiviral antibody responses16,17,18, and VNLRs who showed reduced titers of RBDO-neutralizing antibodies and decreased frequencies of SO-specific CD8+ T cells. Furthermore, VNLRs failed to control delta and omicron infection and COVID-19.
Both the nature and the dose of antigen contribute to the phenotype and the level of heterogeneity of memory and effector T cell subsets25. When we longitudinally analyzed the diversification and turnover rate of S-specific TM cells generated after vaccination, we found predominantly CD4+ and CD8+ TCM1 cell responses to SWT, SB, SD and SO peptides in VNHRs or VR subjects, whereas TEM2 cell responses were prevalent in VNLRs after vaccination. High frequencies of circulating CD8+ TEM1 cells were also reported among multimer-epitope-specific CD8+ T cells in blood up to 120 d after SARS-CoV-2 mRNA vaccination30,41. Our findings suggest that VNHRs could have a wider crossreactive memory T cell repertoire, which can recirculate in tissues and be potentially more responsive after antigen re-encounter. Although T cell responses induced by vaccination or infection can crossrecognize S protein, as also reported by others42,43,44, the frequency of IFN-γ-producing CD4+ T cells and CD8+ T cells were significantly lower in VNLRs compared with the response to SWT. Decreased responses against SO have been also reported in subjects convalescent from SARS-CoV-244.
We used multiparametric flow cytometry to evaluate the phenotype of antigen-specific T cells following S-derived peptide stimulation in vitro. In line with previous studies30,45, the possibility of using overlapping peptide pools, targeting multiple regions in the S protein, offers the unique opportunity to evaluate the overall T cell responses against several epitopes, thus overcoming the limitation of peptide recognition by often unknown polymorphic human leukocyte antigen (HLA) molecules. In fact, peptide–multimer labeling is often restricted to donors expressing the HLA-A2 molecule. However, our approach implies a potential caveat resulting from the possibility that peptide activation could modulate the expression of some differentiation markers expressed by S-specific T cells. The analysis of the immune phenotype of S-specific T cells at steady state will require further deeper investigations and the development of new methodological approaches.
Some similarities between VNHRs and VR subjects support the hypothesis of previous viral exposure46 in VNHRs. In these subjects, vaccination may provide a long-term, polyspecific, immunological memory. Consistent with this hypothesis, robust polyreactive memory CD8+ T cells were reported in a large cohort of hepatitis C virus (HCV)-exposed individuals who did not develop hepatitis and were negative for HCV RNA and anti-HCV antibody46. Pre-existing RNA polymerase-specific T cells have been reported in SARS-CoV-2-exposed individuals, without any evidence of infection47.
Both humoral and cellular immunity protect from SARS-CoV-2 challenge in macaques. CD8+ T cell depletion in infected macaques causes breakthrough virus infection, documented by nasal swabs, after re-challenge with SARS-CoV-248. In this model, viral control postvaccination with adenoviral vectors correlates with both antibody and T cell responses, and with protection against the highly mutated omicron variant12. These findings suggest that CD8+ T cells can continue to be protective at timepoints when neutralizing antibody titers decline or are below the threshold of host protection48. Accordingly, the decreased anti-RBD-binding antibody titers associated with reduced S-specific T cell responses in VNLR subjects could account for the reduced protection observed in this group.
We observed a higher percentage of females among VNHRs compared with VNLRs. Little is known about how sex may impact the immunogenicity and protection elicited by mRNA vaccine. Age- and frailty-associated decrease in humoral responses is greater in males than in females49. The age-related decreases in antibody titers observed in males have been also associated with a decline in CD4+ T cell responses50.
In the present study, we describe three main categories of responders to mRNA vaccination on the basis of S- and RBD-specific IgG titers, S-reactive B cells and polyreactive TM cells. Our results suggest that protection against highly mutated VOCs relied on the contribution of both humoral and cellular immunity. The distinct phenotype and frequency of CD4+ and CD8+ TM cell subsets detected in VNLRs correlated with reduced protection against COVID-19. Further investigations are needed to fully elucidate the mechanisms of differentiation of the various T cell subclasses, and how they evolve postvaccination, in parallel with the clinical outcome. In this context, analysis of the evolution of the TCR repertoire might help elucidate the distinct differentiation paths. Altogether, our observations will contribute to determine protocols to monitor long-term memory responses, and to design new vaccination and targeted booster protocols, especially for at-risk individuals.
Methods
Cohort
In total, 379 staff members of CCI were recruited for the study (Supplementary Table 1). CCI is a certified COVID-free oncological hospital. All the employers have been constantly tested for SARS-CoV-2 infection by PCR following a stringent schedule. Starting from February 2020 to date, each individual has been screened: every 2–4 weeks (subjects were chosen randomly during this timeframe to ensure a daily check in each branch of the hospital); after 5 days out of office; and after close contact with a SARS-CoV-2-infected subject. In case of close contact with an infected subject, participants were isolated in quarantine for 14 days; during this timeframe, they were PCR tested each week until December 2021 or PCR screened every day for 2 weeks (from January 2022). All participants were tested for NWT and SWT IgG levels in plasma at T0.
Participants classified as VN were classified into VNLRs, VNHRs and nonresponders (NRs). NRs were characterized by SWT IgG values <50 a.u. ml−1 at 6 weeks after vaccination and the remaining naive subjects’ IgG values were used to define a threshold to discriminate between VNLRs and VNHRs, leveraging 3 months of IgG data. The distribution of 3-month IgG values for multimodality was tested (P < 2.2 × 10−16, excess mass test) and the antimode (135 a.u. ml−1) was located with the expectation of a bimodal data distribution. This antimode value was used to discriminate between VNLRs and VNHRs (<135 a.u. ml−1 and >135 a.u. ml−1 at 3 months, respectively). In addition, subjects with IgG titers >5 a.u. ml−1 at T0 were defined as seropositive.
Subjects with a documented SARS-CoV-2 infection who recovered from COVID-19 with mild symptoms (subjects with post-acute COVID-19 syndrome, long COVID or lingering symptoms were not included in the study) were classified into VRLRs and VRHRs with the same methodology applied for naive subjects: 3-month IgG values were tested for multimodality (P = 0.49, excess mass test) and split using the antimode 112 a.u. ml−1 located with the expectation of a bimodal data distribution. For the analysis shown in Figs. 2–7, subjects in each class were ranked according to three criteria: (1) subjects were split into three age categories (reported in order of priority for analysis): from 40 years old (y.o.) to 50 y.o., <40 y.o. and >50 y.o.; (2) in these age categories, subjects were ranked by age (from youngest to oldest); and (3) in these age categories, subjects were ranked by measured IgG values at 3 months (from highest to lowest). In addition the gender was also taken into consideration. The top ranking subjects from each class were selected for analysis. According to the SWT IgG quantification and the ranking mentioned above, we decided to focus our analysis on 123 subjects who received the BNT162b2 vaccination (Supplementary Table 2). These subjects were divided into three groups: 43 VNLRs, 42 VNHRs and 38 VR donors. Blood samples were collected before the first BNT162b2 mRNA (Comirnaty, Pfizer Biontech) vaccine dose (T0), before the second vaccine dose (week 3) and after 6 weeks, 3 and 6 months from the first immunization. Blood samples were also collected 1 month after the third vaccine dose (10 months after T0). 96% of donors received BNT162b2 mRNA vaccine as third dose, whereas the remaining 4% received mRNA-1273 Moderna vaccine. Data shown in Fig. 2 to 7 and in Extended Data Fig. 2 to 9 were obtained from donors immunised with BNT162b2 mRNA vaccine. All donors signed informed consent forms approved by the Ethical Committee of the CCI; participants did not receive compensation.
Sample processing
Blood samples were collected into heparin tubes via phlebotomy. Tubes were centrifuged at 800g for 5 min and 4 °C to separate plasma that was used for serological analysis. Whole blood was diluted 1:1 with phosphate-buffered saline (PBS) 1× (Sigma-Aldrich) and PBMC isolation was obtained by density gradient centrifugation using Lympholyte (Cederlane). Tubes were centrifuged at 805g for 30 min at room temperature and PBMCs were collected into new tubes. Cells were washed with PBS 1×, centrifuged at 515g for 10 min and 4 °C, counted with the Burker chamber and cryopreserved in 10% dimethyl sulfoxide in fetal bovine serum (FBS; BioWest).
Spike IgM and IgG quantification
Chemiluminescence immunoassay was performed with TGS COVID-19 Control Set (Technogenetics CVCLCSGM), TGS COVID-19 IgM (CVCL100M) and TGS COVID-19 IgG (CVCL100G) according to the manufacturers’ instructions to quantify IgM and IgG.
Recombinant NWT, SWT-, SD-, SO- and SB-RBD antigen expression and purification
Recombinant SARS-CoV-2 N- and S-RBDs were produced using poly(ethyleneimine)-based transient transfection of Freestyle HEK293 Cells (HEK293-F, Life Technologies) cultivated in suspension. Briefly, cell medium containing the secreted proteins of interest was collected 6 d after transfection by centrifugation at 1,000g for 15 min. The samples were loaded on to a 5-ml His-Trap excel column (Cytiva) using a peristaltic pump and then eluted with 3–250 mM imidazole gradient using an NGC fast protein liquid chromatography system (BioRad). Peak fractions containing the antigens of interest were subjected to immediate concentration with concomitant buffer exchange with fresh PBS to remove imidazole using Amicon centrifugal filters (Merck). Quality controls during protein purification were carried out using reducing and nonreducing sodium dodecylsulfate–polyacryamide gel electrophoresis analysis and differential scanning fluorimetry with a Tycho NT.6 instrument (Nanotemper). All samples were concentrated to 1 mg ml−1, flash-frozen in liquid nitrogen and kept at −80 °C until usage.
ELISA
SARS-CoV-2 N and SARS-CoV-2 S-RBD-specific-binding IgG antibodies were longitudinally tested by ELISA. Maxisorp ELISA 96-well plates (Thermo Fisher Scientific) were coated with 1 µg ml−1 of SARS-CoV-2 nucleocapsid or 2 µg ml−1 of S -RBDWT,-RBDD or -RBDO protein in 1× DPBS and incubated at 4 °C overnight. After incubation, plates were washed 3× with PBS containing 0.1% Tween-20 (PBS-T) and blocked with PBS-T supplemented with 3% bovine serum albumin (BSA; Sigma-Aldrich) for 1 h at room temperature. After washing, plasma was added and incubated for 1.5 h at room temperature. Plasma was diluted in 1% BSA in PBS-T starting from 1:25 or 1:100 dilution and serially diluting each sample by 1:4. Plates were washed 3× with PBS-T and then the secondary antibody (anti-human IgG peroxidase, BD), in 1% BSA and PBS-T, was added and the plates were incubated for 1 h at room temperature. After three washes with PBS-T, plates were developed with TMB Substrate Kit (Thermo Fisher Scientific) at room temperature. The reaction was stopped with 1 M chloridric acid and the plates were read on a Tecan Spark plate reader at 450 nm. Optical densities (ODs) were background subtracted. A positive control standard was created by pooling plasma from six VR subjects, whereas a negative control standard was created by pooling plasma from six pre-pandemic processed plasma samples. Positive and negative control standards were run on each plate. The limit of detection (LOD) was defined as 1:25 for IgG or otherwise stated. The limit of sensitivity (LOS) was established on the basis of uninfected subjects, using plasma from donors never exposed to SARS-CoV-2. For each sample, the ELISA endpoint titer was calculated using nonlinear regression interpolation curve fitting based on the positive control standard. Titers were calculated as the reciprocal serum dilution that yields a corrected OD value of 0.1. Similar results were achieved using Sino Biological SARS-CoV-2 S-RBD proteins.
HEK293TN-hACE2 cell-line generation
HEK293TN-hACE2 (human angiotensin-converting enzyme 2) cell line was generated by lentiviral transduction of HEK293TN cells. HEK293TN cells were obtained from System Bioscience. Lentiviral vectors were produced following a standard procedure based on calcium phosphate co-transfection with third-generation helper and transfer plasmids. The following helper vectors were used (gifts from D. Trono): pMD2.G/VSV-G (Addgene, catalog no. 12259), pRSV-Rev (Addgene, catalog no. 12253) and pMDLg/pRRE (Addgene, catalog no. 12251). The transfer vector pLENTI_hACE2_HygR was obtained by cloning of hACE2 from pcDNA3.1-hACE2 (a gift from F. Li, Addgene, catalog no. 145033) into pLenti-CMV-GFP-Hygro (a gift from E. Campeau and P. Kaufman, Addgene, catalog no. 17446). The hACE2 cDNA was amplified by PCR and inserted under the cytomegalovirus promoter of the pLenti-CMV-GFP-Hygro after green fluorescent protein (GFP) excision with XbaI and SalI digestion; pLENTI_hACE2_HygR is now available through Addgene (catalog no. 155296). After transduction with hACE2 lentiviral vector, cells were subjected to antibiotic selection with hygromycin at 250 μg ml−1. Expression of hACE2 cells was confirmed by flow cytometry staining using anti-hACE2 primary antibody (AF933, R&D Systems) and rabbit anti-goat IgG secondary antibody (Alexa Fluor-647). HEK293TN-hACE2 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% FBS, 1% glutamine, 1% penicillin–streptomycin and 250 μg ml−1 of hygromycin (Gibco) and expression of hACE2 was found to be stable after multiple passages.
Plasmids and molecular cloning
The pCAGGS plasmid containing the sequence encoding for carboxy-terminal, His-tagged Wuhan SARS-CoV-2 Spike RBD (catalog no. NR_52310) was obtained from BEI Resources. Variants were generated by replacing the SARS-Cov-2 Wuhan RBD-encoding sequence with synthetic sequences (Genewiz), encoding for either SARS-CoV-2 RBD delta or SARS-CoV-2 RBD omicron variants into the pCAGGS plasmid template using the 5′-XbaI and 3′-NotI restriction sites.
Production of SARS-CoV-2 pseudoparticles
To generate lentiviral particles pseudotyped with SARS-CoV-2 S, we constructed a series of expression plasmids each encoding a SARS-CoV-2 S mutant. Briefly, for each variant, the corresponding C-terminal-deleted (19 amino acids) S complementary DNA was cloned in pcDNA3.1. Then, pLenti CMV-GFP-TAV2A-LUC Hygro was generated from pLenti-CMV-GFP-Hygro (Addgene, catalog no. 17446) by addition of T2A-Luciferase through PCR cloning. To produce the pseudotyped lentiviral particles, 5 × 106 HEK293TN cells were plated in a 15-cm dish in complete DMEM medium and co-transfected on the following day with 32 µg of plasmid pLenti CMV-GFP-TAV2A-LUC Hygro, 12.5 mg of pMDLg/pRRE (Addgene, catalog no. 12251), 6.25 mg of pRSV-Rev (Addgene, catalog no. 12253) and 9 µg of S plasmid. The medium was replaced with complete Iscove’s modification of DMEM 12 h before transfection. Some 30 h after transfection, the supernatant was collected, clarified by filtration through 0.45-μm pore-size membranes and concentrated by ultracentrifugation (SW32Ti rotor). Viral pseudoparticle suspensions were aliquoted and stored at −80 °C.
Neutralization assay
For the neutralization assay with pseudotyped particles, HEK293TN-hACE2 cells were plated 10,000 per well in white 96-well plates in complete DMEM. After 24 h, cells were transduced with 0.1 multiplicity of infection of SARS-CoV-2 pseudovirus previously incubated with a serial threefold dilution of inactivated plasma to obtain a 7-point dose–response curve. Then, 5 µl of each dilution was added to 45 µl of DMEM containing the pseudovirus and incubated for 1 h at 37 °C. The serum/pseudovirus mixture, 50 µl, was then added to each well and the plates were incubated for 24 h at 37 °C. Each point was assayed in triplicate. After 24 h of incubation, cell transduction was measured by luciferase assay using Bright-Glo Luciferase System (Promega) and Infinite F200 plate reader (Tecan). Measured relative light units were normalized with respect to controls and dose–response curves were generated and neutralization dose 50 (ND50) calculated by nonlinear regression curve fitting with GraphPad Prism.
In vitro stimulation
Frozen PBMCs were thawed in complete medium (RPMI supplemented with 2.5% human serum from Aurogene, 1% l-glutamine, 1% penicillin–streptomycin, 1% nonessential amino acids, 1% sodium pyruvate and 0.1% 2-mercaptoethanol). PBMCs were centrifuged at 515g for 10 min and 4 °C, counted with the Burker chamber and resuspended in complete medium. PBMCs were cultured for either 14 h or 24 h with peptide megapools, prepared as previously described28, consisting in overlapping 15-mers by 10 amino acids covering the complete sequence of the S protein of the WT SARS-CoV-2 (GenBank, accession no. MN_908947) as well as beta, delta and omicron SARS-CoV-2 variants (1 µg ml−1). Complete medium was used as a negative control. Dynabeads human T-activator CD3/CD28 (from Gibco, 1:1 bead:cell ratio) supplemented with human (h)IL-2 (from Novartis, 40 U ml−1) was included as a positive control. To analyze the effector and cytotoxic functions, after 14 h of incubation, PBMCs were stimulated with phorbol 12-myristate 13-acetate (Sigma-Aldrich, 20 ng ml−1) and ionomycin (Sigma-Aldrich, 1 µg ml−1). After 1 h, Brefeldin A (Sigma-Aldrich, 5 µg ml−1) was added and the combined treatment lasted 4 h more. For each condition, triplicate wells containing 3 × 105 cells in 200 µl were plated in 96-well round-bottomed plates and incubated at 37 °C with 5% CO2. The same subjects have been analyzed longitudinally.
Intra-/extracellular staining and flow cytometry analysis
After 14 h of incubation, PBMCs were stained for intracellular cytokines combined with surface markers, whereas after 24 h PBMCs were stained for only surface markers. Cells were washed with 1× PBS supplemented with 0.5% BSA and 2 mM EDTA (FACS buffer) and stained with Fixable Viability Stain 450 in 1× PBS for 20 min at 4 °C to discriminate viable from nonviable cells. Then PBMCs were labeled with the following antibodies for multiparametric flow cytometry analysis: anti-CD3 (BD, SK7, 1:200), anti-CD4 (BD, RPA-T4, 1:400), anti-CD8 (BD, SK1, 1:600), anti-CD14 (BD, MφP9, 1:200), anti-CD16 (BD, 3G8, 1:600), anti-CD19 (BD, HIB19, 1:600), anti-CD25 (BD, 2A3, 1:200), anti-CD27 (BD, M-T271, 1:50), anti-CD45RA (BD, HI100, 1:50), anti-CD45RA (BD, 5H9, 1:200), anti-CD56 (BD, NCAM16.2, 1:200), anti-CD45RO (BD, UCHL-1, 1:200), anti-CD69 (BD, FN50, 1:50), anti-CD197 (BD, 150503, 1:100), anti-IFN-γ (BD, B27, 1:50), anti-IL-2 (BD, MQ1-17H12, 1:100), anti-Granzyme B (BD, GB11, 1:100) and anti-TNF-α (BD, MAb11, 1:100). Dilutions are indicative, because each antibody batch has been titrated. Surface staining was performed in FACS buffer for 30 min at 4 °C. Then, cells were washed and fixed with 1% paraformaldehyde in 1× PBS. For the intracellular cytokines staining, after fixation, cells were permeabilized with 1× Perm/Wash buffer (BD) according to the manufacturer’s instructions and stained for 1 h at room temperature in BD Perm/Wash buffer. Cells were then washed twice and resuspended in FACS buffer for data acquisition. The same subjects have been analyzed longitudinally. Flow cytometry data were acquired on a BD LSR Fortessa X-20 instrument and analyzed with FlowJo software. The LOD for antigen-specific CD4+ and CD8+ T cell responses was calculated as the geomean twofold s.d. from the negative control (unstimulated). The LOS for antigen-specific CD4+ and CD8+ T cell responses was calculated as the median twofold s.d. from the negative control (unstimulated)28. When LOD and LOS were negative or 0, we considered as active responses only those >0.01 after background subtraction. No detected responses are labeled ND.
Detection of SARS-CoV-2 antigen-specific B cells
The analysis of SARS-CoV-2 antigen-specific B cells was performed using the SARS-CoV-2 B Cell Analysis Kit (Miltenyi) according to the manufacturer’s instructions. PBMCs were labeled with two tetramers formed from a recombinant SARS-CoV-2 SWT protein (conjugated with phycoerythrin (PE) and PE-Vio), combined with the following antibodies: anti-CD19, anti-CD27, anti-IgG, anti-IgA and anti-IgM.
Analysis of VOC prevalence
Aggregated GISAID (https://doi.org/10.1002/gch2.1018) and aggregated COVID-19 case data for the percentage distribution of VOCs in Italy was downloaded from the European Centre for Disease Prevention and Control (https://www.ecdc.europa.eu/en/publications-data/data-virus-variants-covid-19-eueea; access date 15 April 2022).
Statistical analysis
Wilcoxon’s signed-rank (nonparametric, paired), Wilcoxon’s rank-sum (nonparametric, unpaired) and Spearman’s correlation tests were used for statistical analysis and the P value was determined using Prism software (Graphpad Software, Inc.), or otherwise as indicated in the text. Significance of ratio was assessed by Wilcoxon’s signed-rank t-test compared with a hypothetical median of 1. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
References
Fisher, D. & Wilder-Smith, A. The global community needs to swiftly ramp up the response to contain COVID-19. Lancet 395, 1109–1110 (2020).
Celardo, I., Pace, L., Cifaldi, L., Gaudio, C. & Barnaba, V. The immune system view of the coronavirus SARS-CoV-2. Biol. Direct 15, 30 (2020).
Thomas, S. J. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N. Engl. J. Med. 385, 1761–1773 (2021).
Moderna. Moderna Reports Second Quarter Fiscal Year 2021 Financial Results and Provides Business Updates. Press release (2021).
Regev-Yochay, G. et al. Decreased infectivity following BNT162b2 vaccination: a prospective cohort study in Israel. Lancet Reg. Health Eur. 7, 100150 (2021).
Baden, L.R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Pegu, A. et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 373, 1372–1377 (2021).
Altmann, D. M. & Boyton, R. J. SARS-CoV-2 T cell immunity: specificity, function, durability, and role in protection. Sci. Immunol. 5, eabd6160 (2020).
Channappanavar, R., Fett, C., Zhao, J., Meyerholz, D. K. & Perlman, S. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J. Virol. 88, 11034–11044 (2014).
Gallais, F. et al. Intrafamilial exposure to SARS-CoV-2 associated with cellular immune response without seroconversion, France. Emerg. Infect. Dis. 27, 113–121 (2021).
Chandrashekar, A. et al. Vaccine protection against the SARS-CoV-2 omicron variant in macaques. Cell 185, 1549–1555e1511 (2022).
Abu-Raddad, L. J. et al. SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy. eClinicalMedicine 35, 100861 (2021).
Hall, V. J. et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet 397, 1459–1469 (2021).
Lumley, S. F. et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N. Engl. J. Med. 384, 533–540 (2021).
Barda, N. et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N. Engl. J. Med. 385, 1078–1090 (2021).
Schmidt, F. et al. High genetic barrier to SARS-CoV-2 polyclonal neutralizing antibody escape. Nature 600, 512–516 (2021).
Stamatatos, L. et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science 372, 1413–1418 (2021).
Rodda, L. B. et al. Imprinted SARS-CoV-2-specific memory lymphocytes define hybrid immunity. Cell 185, 1588–1601.e14 (2022).
Gaebler, C. et al. Evolution of antibody immunity to SARS-CoV-2. Nature 591, 639–644 (2021).
Wang, Z. et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 595, 426–431 (2021).
Anderson, E. J. et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 383, 2427–2438 (2020).
Sahin, U. et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 586, 594–599 (2020).
Lederer, K. et al. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity 53, 1281–1295.e1285 (2020).
Pace, L. Temporal and epigenetic control of plasticity and fate decision during CD8+ T-cell memory differentiation. Cold Spring Harb. Perspect. Biol. 13, a037754 (2021).
Jarjour, N. N., Masopust, D. & Jameson, S. C. T cell memory: understanding COVID-19. Immunity 54, 14–18 (2021).
Jameson, S. C. & Masopust, D. Understanding subset diversity in T cell memory. Immunity 48, 214–226 (2018).
Tarke, A. et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from alpha to omicron. Cell 185, 847–859.e811 (2022).
Mateus, J. et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science 374, eabj9853 (2021).
Goel, R. R. et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 374, eabm0829 (2021).
Cao, Y. et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 602, 657–663 (2022).
Cele, S. et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 602, 654–656 (2022).
Grifoni, A. et al. SARS-CoV-2 human T cell epitopes: aaptive immune response against COVID-19. Cell Host Microbe 29, 1076–1092 (2021).
Romero, P. et al. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J. Immunol. 178, 4112–4119 (2007).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Mathew, D. et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 369, eabc8511 (2020).
Giles, J. R. et al. Human epigenetic and transcriptional T cell differentiation atlas for identifying functional T cell-specific enhancers. Immunity 55, 557–574.e557 (2022).
Braun, J. et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 587, 270–274 (2020).
Grifoni, A. et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 181, 1489–1501.e1415 (2020).
Sekine, T. et al. Robust T cell immunity in convalescent Individuals with asymptomatic or mild COVID-19. Cell 183, 158–168.e114 (2020).
Oberhardt, V. et al. Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine. Nature 597, 268–273 (2021).
Lan, J. et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581, 215–220 (2020).
GeurtsvanKessel, C. H. et al. Divergent SARS-CoV-2 omicron-reactive T and B cell responses in COVID-19 vaccine recipients. Sci. Immunol. 7, eabo2202 (2022).
Keeton, R. et al. T cell responses to SARS-CoV-2 spike cross-recognize omicron. Nature 603, 488–492 (2022).
Mateus, J. et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 370, 89–94 (2020).
Scognamiglio, P. et al. Presence of effector CD8+ T cells in hepatitis C virus-exposed healthy seronegative donors. J. Immunol. 162, 6681–6689 (1999).
Swadling, L. et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature 601, 110–117 (2021).
McMahan, K. et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 590, 630–634 (2021).
Shapiro, J. R. et al. Association of frailty, age, and biological sex with SARS-CoV-2 mRNA vaccine-induced immunity in older adults. Clin. Infect. Dis. 75(Suppl. 1), S61–S71 (2022).
Bai, J. et al. Sex, age, and ethnic background shape adaptive immune responses induced by the SARS-CoV-2 mRNA vaccine. Front. Immunol. 13, 786586 (2022).
Acknowledgements
We thank M. Olivero, L. Palmas, R. Albano and D. Baev for helpful discussion and technical support, the CCI and AVIS volunteers for donating their blood and the nurses for their assistance. We thank all the IIGM and CCI colleagues for helpful discussions and all technicians for help. We thank A. Pinnola for support with molecular cloning and A. Canciani for support with antigen purification. L.P. received founding from IIGM/CSP, Armenise-Harvard Foundation, Ministero della Salute ‘COVID-2020–12371849’, FPO/Candiolo Advance 5 × 1000_2018 ‘Im-MEMORY’; A. Sapino received funding from FPRC 5 × 1,000 M.I.U.R 2017 ‘INTERONC-EMALAB’, FPO 5 × 1000 Ministero Salute 2018 ‘EMALAB’, Ministero Salute Ricerca Corrente 2021. F.F. received funding from Armenise-Harvard Foundation, AIRC ‘MFAG 20075’, NATO ‘SPS G5701’, Velux Stiftung ‘1375’, Mizutani Foundation for Glycoscience ‘200039’ and MUR (Dipartimenti di Eccellenza 2018–2022); and obtained funding through BEI Resources, the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH): vector pCAGGS containing the SARS-related coronavirus 2, Wuhan-Hu-1 spike glycoprotein receptor-binding domain (RBD) (no. NR-52309). A.Sette received founding from federal funds from the NIAID, NIH and Department of Health and Human Services under contract no. 75N93021C00016, and V.B. from AIRC (no. IG-2017 ID 19939).
Author information
Authors and Affiliations
Contributions
L.P., A. Sottile, A. Sapino and V.B. conceived the project. L.P., A. Sottile, A. Sapino, V.B., I.E., N.B., G.M., S.A.S., V.R., C.DI., K.G., D.B., F.F. A.G., A.S., S.M., R.D., S.M. and F.F. performed the methodology. I.E., N.B., G.M., S.A.S., V.R., C.DI., K.G., D.B., F.F., M. Macagno, M. Montone and B.M. carried out the investigations. L.P., A. Sottile, A. Sapino, V.B., I.E., N.B., G.M., S.A.S., V.R. and C.D.I. visualized the study. L.P., A. Sottile, A. Sapino and V.B. acquired the funding. L.P., A. Sottile and A. Sapino administered the project. L.P., A. Sottile and A. Sapino supervised the project. L.P., V.B., A. Sapino, I.E., S.A.S., V.R. and C.D.I. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.Sette is a consultant for Gritstone Bio, Flow Pharma, Arcturus Therapeutics, ImmunoScape, CellCarta, Avalia, Moderna, Fortress and Repertoire. All other authors declare no conflicts of interest. L.J.I. has filed for patent protection for various aspects of T cell epitope and vaccine design work. CCI. has filed a patent for immune correlate of protection after RNA vaccination. R.D.F. is a consultant for Moderna Inc. The data are tabulated in the main paper and in the supplementary materials.
Peer review
Peer review information
Nature Immunology thanks Tao Dong, Mala Maini and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor:Ioana Visan, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Longitudinal analysis of humoral responses to BNT162b2 mRNA vaccine.
a, ELISA showing NWT antibody titers of SARS-CoV-2 naïve (n = 331) and SARS-CoV-2 recovered V (n = 48) subjects measured at T0. All data are shown as ELISA ETs based on a standard. The dotted line indicates LOD. b, CLIA analysis showing SWT IgM titers in naive, seropositive and recovered subjects at T0, weeks 3 and 6 and months 3 and 6.NR subjects are also reported.c, CLIA analysis showing SWT IgG titers in VR subjects divided in VRLRs and VRHRs at T0, weeks 3 and 6 and months 3 and 6. d, CLIA analysis showing SWT IgG titers in VN (LRs and HRs) and VR (VRLRs and VRHRs) subjects at month 6 vs subjects’ age. e, CLIA analysis showing SWT IgG titers in VN (VNLR and VNHR) and VR (VRLR and VRHR) subjects at month 6 grouped by age range. The same subjects were longitudinally analyzed. Boxes show median, upper and lower quartiles, and whiskers show 1.5x the interquartile range (IQR) on either side (panels b,c,e) or minimum/maximum (panel a). Each dot represents one subject. Each dot of box plots represents the average between two technical replicates of the same subject. All data are pooled. Statistics were calculated using two-sided Wilcoxon rank-sum test (panels a,c,e) or signed-rank test (panel b), with Benjamini Hochberg correction for multiple testing. **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.
Extended Data Fig. 2 Analysis of B cell responses to SARS-CoV-2 BNT162b2 mRNA vaccine.
a, Gating strategy ancestry: lymphocytes (FSC-A/SSC-A), FSC singlets (FSC-W/FSC-A), SSC singlets (SSC-W/SSC-A), live cells (PerCp−Cy5.5-7-AAD/FSC-A), CD19+ cells (APC-Cy7-CD19/FSC-A), SWT cells (PE-Vio-SWT tetramer/PE-SWT tetramer). IgM vs CD27 (APC-IgM/FITC-CD27), IgG vs CD27 (BV421-IgG/FITC-CD27), IgA vs CD27 (BV510-IgA/FITC-CD27) on gated CD19+ cells (positive control) and on gated SWT+ CD19+ B cells. b, Flow cytometry analysis showing the percentage of CD19+ B cells in VNLR, VNHR and VR subjects at month 6. c, Representative flow cytometry dot plots of SWT-specific tetramer+ CD19+B cells from VNLR, VNHR and VR subjects at T0. d, Flow cytometry analysis showing the percentage of CD27+ IgM+ SWT- tetramer+ B cells in VNLR, VNHR and VR subjects at T0, week 6 and month 6. Below: percentages of VNLR, VNHR and VR subjects with active responses > 0.01. e, Flow cytometry analysis showing the cell number of CD27+ IgA+ SWT- tetramer+ B cells per 106 PBMCs in VNLR, VNHR and VR subjects at T0, week 6 and month 6. At T0: VNLR (n = 25), VNHR (n = 24), VR subjects (n = 12). At week 6: VNLR (n = 12), VNHR (n = 17), VR subjects (n = 10). At month 6: VNLR (n = 26), VNHR (n = 29), VR subjects (n = 22). The same subjects were longitudinally analyzed. Box plots indicate median, interquartile range and minimum/maximum. Each dot of box plots represents the average between three technical replicates of the same subject. All data are pooled from multiple experiments. The dashed black line indicates LOD. Statistics were calculated using two-sided Wilcoxon rank-sum tests. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Extended Data Fig. 3 Analysis of anti S-RBDWT -RBDD and -RBDO binding Abs.
a,ELISA titration analysis showing S-RBDWT, -RBDD and -RBDO IgG from representative VNLRs (n = 3), VNHRs (n = 3), and VR subjects (n = 3) at month 3. For S-RBDWT IgG, plasma was diluted starting with 1:25 dilution, followed by 1:4 serial dilutions for all subjects. For S-RBDD and S-RBDO IgG, plasma was diluted starting with 1:25 dilution followed by 1:4 serial dilutions for VNLRs or starting with 1:100 dilution followed by 1:4 serial dilutions forVNHRs and VR subjects.
Extended Data Fig. 4 Flow cytometry gating strategy.
a,Gating strategy ancestry: lymphocytes (FSC-A/SSC-A), FSC singlets (FSC-W/FSC-A), SSC singlets (SSC-W/SSC-A), Dump- live cells [Dead FVS450+ cells and CD14+ CD16+ CD19+ CD56+ (Dump+) cells excluded], CD4 vs CD8 (BV605-CD8/BV786-CD4). b, c, Differentiation markers on CD4+ (b) or CD8+ (c)T cells. CD4+ or CD8+ T cells, CD25 vs CD69 (BV-510-CD25/APC-R700-CD69). CD4+ or CD8+ T cells with high gMFI levels for both CD25 and CD69 were gated as double positive cells. CD45RA (APC-CD45RA/FSC-A) on gated CD4+ or CD8+ and on gated CD25+ CD69+ CD4+ or CD8+ T cells, CCR7 vs CD27 (BV711-CCR7/ APC-H7-CD27) on gated CD45RA− and on gated CD45RA+ cells. d, e, Flow cytometry analysis showing the percentage of CD4+ (d) and CD8+ (e) T cells in VNLRs (n = 25), VNHRs (n = 24), and VR subjects (n = 12) at month 6. f, g, Representative flow cytometry dot plots of CD25 vs CD69 cells on gated CD4+ (f) and CD8+ (g) T cells from VNLRs, VNHRs and VR subjects at T0, after 24h incubation with Medium (Unstimulated) or SWT. Data are represented with background subtraction from paired unstimulated controls. The same subjects were longitudinally analyzed. Box plots indicate median, interquartile range, and minimum/maximum. Each dot of box plots represents the average between three technical replicates of the same subject. All data are pooled from multiple experiments. Statistics were calculated using two-sided Wilcoxon rank-sum tests.
Extended Data Fig. 5 Analysis of specific CD4+ and CD8+ T cell responses against SWT, SD, SO and SB in VNLR, VNHR and VR subjects.
a, b, Flow cytometry analysis showing the percentage of CD25+ CD69+ CD4+ (a) and CD25+ CD69+ CD8+ (b) T cells in VNLRs (n = 25), VNHRs (n = 24), and VR subjects (n = 12) at T0 and month 6, after 24h incubation with SWT, SD, SO or SB peptide megapools. c,d, Flow cytometry analysis showing the percentage of CD25+ CD69+ CD4+ (c) and CD25+ CD69+ CD8+ (d) T cells from VNLRs (n = 25), VNHRs (n = 24), and VR subjects (n = 12) at T0 and month 6, after 24h incubation with anti (α) -CD3 plus CD28 beads and IL-2. Data are represented with background subtraction from paired unstimulated controls. The same subjects were longitudinally analyzed. Each dot of box plots represents the average between three technical replicates of the same subject. All data are pooled from multiple experiments. Statistics were calculated using two-sided Wilcoxon signed-rank tests. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Extended Data Fig. 6 Analysis of effector molecules against SWT, SD, SO and SB in VNLR, VNHR and VR subjects.
a, Gating strategy ancestry: lymphocytes (FSC-A/SSC-A), FSC singlets (FSC-W/FSC-A), SSC singlets (SSC-W/SSC-A), CD3+ Dump− live cells (BUV805-CD3/BV421-Dump) [Dead FVS450+ cells and CD14+ CD16+ CD19+ CD56+ (Dump+) cells excluded], CD4 vs CD8 (BV605-CD8/BV786-CD4). Intracellular effector molecules: IFN-γ (Pe-Cy7-IFN-γ/FSC-A), IL-2 (PE-IL-2/FSC-A) and TNF-α (PerCP-Cy5.5-TNFα-/FSC-A) on gated CD4+ and on gated CD8+ T cells. b, c, Flow cytometry analysis showing the percentage of IL-2+ (b) and TNF-α+ (c) cells on gated CD4+ and CD8+ T cells in VNLRs (n = 25), VNHRs (n = 24), and VR subjects (n = 12) subjects at month 6, after 14h incubation with SWT,SD, SO or SB peptide megapools. Below: percentages of subjects with active responses > 0.01. d, Flow cytometry analysis showing the percentage of IFN-γ+, IL-2+ and TNF-α+ cells on gated CD4+ and CD8+ T cells from VNLRs (n = 25), VNHRs (n = 24), and VR subjects (n = 12) subjects at month 6, after 14h incubation with α -CD3 plus -CD28 beads and IL-2. Data are represented with background subtraction from paired unstimulated controls. The same subjects were longitudinally analyzed. Box plots indicate median, interquartile range, and minimum/maximum. Each dot of box plots represents the average between three technical replicates of the same subject. All data are pooled from multiple experiments. Statistics were calculated using two-sided Wilcoxon rank-sum tests. ***P < 0.001.
Extended Data Fig. 7 Analysis of specific CD4+ and CD8+ T cell responses against SWT, SD, SO and SB in VNLR, VNHR and VR subjects.
a-f, Flow cytometry analysis showing the percentage of CD25+ CD69+ CD4+ TCM1 (a), TEM1 (b), TEM2 (c) cells and CD25+ CD69+ CD8+ TCM1 (d), TEM1 (e), TEM2 (f) cells in VNLRs (n = 25), VNHRs (n = 24), and VR subjects (n = 12) at T0 and month 6, after 24h incubation with SWT, SD, SO or SB peptide megapools. Data are represented with background subtraction from paired unstimulated controls. The same subjects were longitudinally analyzed. Each dot of box plots represents the average between three technical replicates of the same subject. All data are pooled from multiple experiments. Statistics were calculated using two-sided Wilcoxon signed-rank tests. *P < 0.05; **P < 0.01; ***P < 0.001; ND = not detected.
Extended Data Fig. 8 Polyreactive CD4+ and CD8+ T cell responses in VNLR, VNHR and VR subjects.
a-f, Flow cytometry analysis showing the percentage of CD25+CD69+ TCM1 (a, b) TEM1 (c, d) and TEM2 (e, f) cells on gated CD4+ (a, c, e) and CD8+ (b, d, f) T cells from VNLRs (n = 25), VNHRs (n = 24), and VR subjects (n = 12) at T0 and month 6, after 24h incubation with α-CD3 plus -CD28 beads and IL-2. The dotted black lines indicate the LOD. The dotted green lines indicate the LOS. The same subjects were longitudinally analyzed. Data are represented with background subtraction from paired unstimulated controls. Box plots indicate median, interquartile range, and minimum/maximum. Each dot of box plots represents the average between three technical replicates of the same subject. All data are pooled from multiple experiments. Statistics were calculated using two-sided Wilcoxon rank-sum tests.
Extended Data Fig. 9 Analysis of S-specific TN, TCM2, and TEMRA cell responses on gated CD25+ CD69+ CD4+ and CD8+ T cells.
a-f, Flow cytometry analysis showing the percentage of TN (a, d), TCM2 (b, e) and TEMRA (c, f) cells on gated CD25+ CD69+ CD4+ (a-c) and CD8+ (d-f) T cells from VNLRs (n = 25), VNHRs (n = 24), and VR subjects (n = 12) at T0 and month 6, after 24h incubation with SWT,SD, SO or SB peptide megapools. The dotted black lines indicate the LOD. The dotted green lines indicate the LOS. Below: percentages of subjects with active responses > 0.01. Data are represented with background subtraction from paired unstimulated controls. The same subjects were longitudinally analyzed. Box plots indicate median, interquartile range, and minimum/maximum. Each dot of box plots represents the average between three technical replicates of the same subject. All data are pooled from multiple experiments. Statistics were calculated using two-sided Wilcoxon rank-sum tests. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Extended Data Fig. 10 Analysis of specific CD4+ and CD8+ T cell responses against SWT, SD, SO and SB in VNLR, VNHR and VR subjects, at months 6 and 10 postvaccination.
a, b, Flow cytometry analysis showing the percentage of CD25+ CD69+ CD4+ (a) and CD8+ (b) T cells in VNLRs (n = 13), and VNHRs (n = 12) at months 6 and 10, after 24h incubation with SWT, SD, SO or SB peptide megapools. The same subjects were longitudinally analyzed. Data are represented with background subtraction from paired unstimulated controls. Each dot of box plots represents the average between three technical replicates of the same subject. All data are pooled from multiple experiments. Statistics were calculated using two-sided Wilcoxon signed-rank tests. *P < 0.05.
Supplementary information
Supplementary Information
Supplementary Tables 1–3.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brasu, N., Elia, I., Russo, V. et al. Memory CD8+ T cell diversity and B cell responses correlate with protection against SARS-CoV-2 following mRNA vaccination. Nat Immunol 23, 1445–1456 (2022). https://doi.org/10.1038/s41590-022-01313-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-022-01313-z
This article is cited by
-
A quest for universal anti-SARS-CoV-2 T cell assay: systematic review, meta-analysis, and experimental validation
npj Vaccines (2024)
-
Three immunizations with Novavax’s protein vaccines increase antibody breadth and provide durable protection from SARS-CoV-2
npj Vaccines (2024)
-
Fc-γR-dependent antibody effector functions are required for vaccine-mediated protection against antigen-shifted variants of SARS-CoV-2
Nature Microbiology (2023)
-
Un nuovo test COVID può dire chi ha più bisogno di un richiamo
Nature Italy (2022)
-
A new COVID test can tell who needs a booster shot first
Nature Italy (2022)