Abstract
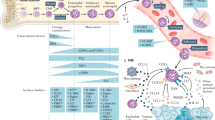
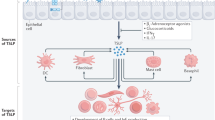
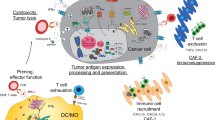
Eosinophils are important effector cells and therapeutic targets in allergic diseases. Emerging data indicate that eosinophils infiltrate a variety of solid tumor types and have pleiotropic activities by at least two non-mutually exclusive mechanisms: direct interactions with tumor cells, and intricate cross-talk with lymphocytes. In light of the immune checkpoint inhibition revolution in cancer therapy, we review eosinophil–lymphocyte interactions in the tumor microenvironment. We also analyze potential interactions between eosinophils and lymphocyte subsets, including T cells, natural killer cells and innate lymphoid cells. We provide perspectives on the consequences of these interactions and how eosinophils are accessory cells that can affect the response to various forms of T cell-mediated immunotherapies and might be therapeutically targeted to improve cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jacobsen, E. A. et al. Eosinophil knockout humans: uncovering the role of eosinophils through eosinophil-directed biological therapies. Annu. Rev. Immunol. 39, 719–757 (2021).
Reinbach, G. Ueber das Verhalten der Leukocyten bei malignen Tumoren. Arch. Klin. Chir. Arch. Klin. Chir. 46, 486–562 (1893).
Grisaru-Tal, S. et al. Primary tumors from mucosal barrier organs drive unique eosinophil infiltration patterns and clinical associations. Oncoimmunology 10, 1859732 (2020).
Grisaru-Tal, S., Itan, M., Klion, A. D. & Munitz, A. A new dawn for eosinophils in the tumour microenvironment. Nat. Rev. Cancer 20, 594–607 (2020).
Jacquelot, N., Seillet, C., Vivier, E. & Belz, G. T. Innate lymphoid cells and cancer. Nat. Immunol. 23, 371–379 (2022).
Rodriguez-Rodriguez, N., Gogoi, M. & McKenzie, A. N. J. Group 2 innate lymphoid cells: team players in regulating asthma. Annu. Rev. Immunol. 39, 167–198 (2021).
Nussbaum, J. C. et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502, 245–248 (2013).
Maggi, E., Veneziani, I., Moretta, L., Cosmi, L. & Annunziato, F. Group 2 innate lymphoid cells: a double-edged sword in cancer? Cancers 12, 3452 (2020).
Ikutani, M. et al. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J. Immunol. 188, 703–713 (2012).
Jacquelot, N. et al. Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma. Nat. Immunol. 22, 851–864 (2021).
Dankort, D. et al. BrafV600E cooperates with Pten loss to induce metastatic melanoma. Nat. Genet. 41, 544–552 (2009).
Dougan, M., Dranoff, G. & Dougan, S. K. GM-CSF, IL-3 and IL-5 family of cytokines: regulators of inflammation. Immunity 50, 796–811 (2019).
Martin, N. T. & Martin, M. U. Interleukin 33 is a guardian of barriers and a local alarmin. Nat. Immunol. 17, 122–131 (2016).
Lucarini, V. et al. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology 6, e1317420 (2017).
Gao, K. et al. Transgenic expression of IL-33 activates CD8+ T cells and NK cells and inhibits tumor growth and metastasis in mice. Cancer Lett. 335, 463–471 (2013).
Andreone, S. et al. IL-33 promotes CD11b/CD18-mediated adhesion of eosinophils to cancer cells and synapse-polarized degranulation leading to tumor cell killing. Cancers 11, 1664 (2019).
Brusilovsky, M. et al. Environmental allergens trigger type 2 inflammation through ripoptosome activation. Nat. Immunol. 22, 1316–1326 (2021).
Munitz, A. et al. 2B4 (CD244) is expressed and functional on human eosinophils. J. Immunol. 174, 110–118 (2005).
Munitz, A. et al. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF and eotaxin on human peripheral blood eosinophils. Blood 107, 1996–2003 (2006).
Pesce, S. et al. The innate immune cross-talk between NK cells and eosinophils is regulated by the interaction of natural cytotoxicity receptors with eosinophil surface ligands. Front. Immunol. 8, 510 (2017).
Qi, L. et al. Interleukin-33 activates and recruits natural killer cells to inhibit pulmonary metastatic cancer development. Int. J. Cancer 146, 1421–1434 (2020).
O’Flaherty, S. M. et al. TLR-stimulated eosinophils mediate recruitment and activation of NK cells in vivo. Scand. J. Immunol. 85, 417–424 (2017).
Schuijs, M. J. et al. ILC2-driven innate immune checkpoint mechanism antagonizes NK cell antimetastatic function in the lung. Nat. Immunol. 21, 998–1009 (2020).
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 (2020).
Tay, R. E., Richardson, E. K. & Toh, H. C. Revisiting the role of CD4+ T cells in cancer immunotherapy—new insights into old paradigms. Cancer Gene Ther. 28, 5–17 (2021).
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Oh, D. Y. & Fong, L. Cytotoxic CD4+ T cells in cancer: expanding the immune effector toolbox. Immunity 54, 2701–2711 (2021).
Grisaru-Tal, S. et al. Metastasis-entrained eosinophils enhance lymphocyte-mediated antitumor immunity. Cancer Res. 81, 5555–5571 (2021).
Mattes, J. et al. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J. Exp. Med. 197, 387–393 (2003).
Hung, K. et al. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 188, 2357–2368 (1998).
Reichman, H. et al. Activated eosinophils exert antitumorigenic activities in colorectal cancer. Cancer Immunol. Res. 7, 388–400 (2019).
Dolitzky, A. et al. Transcriptional profiling of mouse eosinophils identifies distinct gene signatures following cellular activation. Front. Immunol. 12, 802839 (2021).
Carretero, R. et al. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8+ T cells. Nat. Immunol. 16, 609–617 (2015).
Akuthota, P., Wang, H. B., Spencer, L. A. & Weller, P. F. Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin. Exp. Allergy 38, 1254–1263 (2008).
Munitz, A. et al. CD48 is an allergen and IL-3-induced activation molecule on eosinophils. J. Immunol. 177, 77–83 (2006).
Arnold, I. C. et al. Eosinophils suppress TH1 responses and restrict bacterially induced gastrointestinal inflammation. J. Exp. Med. 215, 2055–2072 (2018).
Woerly, G. et al. Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon gamma): inhibition by immunoglobulin a complexes. J. Exp. Med. 190, 487–495 (1999).
Onyema, O. O. et al. Eosinophils downregulate lung alloimmunity by decreasing TCR signal transduction. JCI Insight 4, e128241 (2019).
Lucey, D. R., Nicholson-Weller, A. & Weller, P. F. Mature human eosinophils have the capacity to express HLA-DR. Proc. Natl Acad. Sci. USA 86, 1348–1351 (1989).
Hansel, T. T. et al. Sputum eosinophils from asthmatics express ICAM-1 and HLA-DR. Clin. Exp. Immunol. 86, 271–277 (1991).
Akuthota, P., Wang, H. & Weller, P. F. Eosinophils as antigen-presenting cells in allergic upper airway disease. Curr. Opin. Allergy Clin. Immunol. 10, 14–19 (2010).
Gurtner, A. et al. Single-cell RNA sequencing unveils intestinal eosinophil development and specialization. Preprint at bioRxiv https://doi.org/10.1101/2021.10.27.466053 (2021).
Lee, J. J. et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 305, 1773–1776 (2004).
Arnold, I. C. et al. The GM-CSF–IRF5 signaling axis in eosinophils promotes antitumor immunity through activation of type 1 T cell responses. J. Exp. Med. 217, e20190706 (2020).
Jia, S., Li, W., Liu, P. & Xu, L. X. A role of eosinophils in mediating the anti-tumour effect of cryo-thermal treatment. Sci. Rep. 9, 13214 (2019).
Fallegger, A. et al. TGF-beta production by eosinophils drives the expansion of peripherally induced neuropilin −RORγt+ regulatory T cells during bacterial and allergen challenge. Mucosal. Immunol. 15, 504–514 (2022).
Zaynagetdinov, R. et al. Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment. Cancer Res. 75, 1624–1634 (2015).
Sharma, P. & Allison, J. P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214 (2015).
Webster, R. M. The immune checkpoint inhibitors: where are we now? Nat. Rev. Drug Discov. 13, 883–884 (2014).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Simon, H. U. et al. Interleukin-2 primes eosinophil degranulation in hypereosinophilia and Wells’ syndrome. Eur. J. Immunol. 33, 834–839 (2003).
Sosman, J. A. et al. Evidence for eosinophil activation in cancer patients receiving recombinant interleukin-4: effects of interleukin-4 alone and following interleukin-2 administration. Clin. Cancer Res. 1, 805–812 (1995).
Ellem, K. A. et al. A case report: immune responses and clinical course of the first human use of granulocyte/macrophage-colony-stimulating-factor-transduced autologous melanoma cells for immunotherapy. Cancer Immunol. Immunother. 44, 10–20 (1997).
Gebhardt, C. et al. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin. Cancer Res. 21, 5453–5459 (2015).
Martens, A. et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin. Cancer Res. 22, 2908–2918 (2016).
Lang, B. M. et al. Long-term survival with modern therapeutic agents against metastatic melanoma—vemurafenib and ipilimumab in a daily life setting. Med. Oncol. 35, 24 (2018).
Simon, S. C. S. et al. Eosinophil accumulation predicts response to melanoma treatment with immune checkpoint inhibitors. Oncoimmunology 9, 1727116 (2020).
Cruikshank, W. & Center, D. M. Modulation of lymphocyte migration by human lymphokines. II. Purification of a lymphotactic factor (LCF). J. Immunol. 128, 2569–2574 (1982).
Zheng, X. et al. CTLA4 blockade promotes vessel normalization in breast tumors via the accumulation of eosinophils. Int. J. Cancer 146, 1730–1740 (2020).
Moreira, A., Leisgang, W., Schuler, G. & Heinzerling, L. Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy. Immunotherapy 9, 115–121 (2017).
Weide, B. et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin. Cancer Res. 22, 5487–5496 (2016).
Alves, A., Dias, M., Campainha, S. & Barroso, A. Peripheral blood eosinophilia may be a prognostic biomarker in non-small cell lung cancer patients treated with immunotherapy. J. Thorac. Dis. 13, 2716–2727 (2021).
Herrmann, T. et al. Eosinophil counts as a relevant prognostic marker for response to nivolumab in the management of renal cell carcinoma: a retrospective study. Cancer Med. 10, 6705–6713 (2021).
Ghebeh, H., Elshenawy, M. A., AlSayed, A. D. & Al-Tweigeri, T. Peripheral blood eosinophil count is associated with response to chemoimmunotherapy in metastatic triple-negative breast cancer. Immunotherapy 14, 189–199 (2022).
Nishikawa, D. et al. Eosinophil prognostic scores for patients with head and neck squamous cell carcinoma treated with nivolumab. Cancer Sci. 112, 339–346 (2021).
Hollande, C. et al. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat. Immunol. 20, 257–264 (2019).
Sterner, R. C. & Sterner, R. M. CAR T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021).
Jia, Q. et al. Peripheral eosinophil counts predict efficacy of anti-CD19 CAR-T cell therapy against B-lineage non-Hodgkin lymphoma. Theranostics 11, 4699–4709 (2021).
Cheng, J. N. et al. Radiation-induced eosinophils improve cytotoxic T lymphocyte recruitment and response to immunotherapy. Sci. Adv. 7, eabc7609 (2021).
Lai, W. et al. Human pluripotent stem cell-derived eosinophils reveal potent cytotoxicity against solid tumors. Stem Cell Rep. 16, 1697–1704 (2021).
Li, M. O. et al. Innate immune cells in the tumor microenvironment. Cancer Cell 39, 725–729 (2021).
Rafei-Shamsabadi, D., Lehr, S., Behrens, M. & Meiss, F. Additive intralesional interleukin-2 improves progression-free survival in a distinct subgroup of melanoma patients with prior progression under immunotherapy. Cancers 14, 540 (2022).
Hude, I. et al. Leucocyte and eosinophil counts predict progression-free survival in relapsed or refractory classical Hodgkin lymphoma patients treated with PD1 inhibition. Br. J. Haematol. 181, 837–840 (2018).
Furubayashi, N. et al. The association of clinical outcomes with posttreatment changes in the relative eosinophil counts and neutrophil-to-eosinophil ratio in patients with advanced urothelial carcinoma treated with pembrolizumab. Cancer Manag. Res. 13, 8049–8056 (2021).
Acknowledgements
This work was supported by grants and fellowships to A.M. from the US-Israel Bi-national Science Foundation (grant no. 2015163, to A.M. and M.E.R.), Israel Science Foundation (grant nos. 886/15 and 542/20), Israel Cancer Research Fund, Richard Eimert Research Fund on Solid Tumors, Israel Cancer Association, Dotan Hemato Oncology fund, Cancer Biology Research Center, Tel Aviv University, The Tel Aviv University Faculty of Medicine Recanati Fund and Azrieli Foundation Canada-Israel. M.E.R. was further supported by the National Institutes of Health (R37 AI045898, R01 AI124355, U19 AI070235 and P30 DK078392; Gene and Protein Expression Core), Campaign Urging Research for Eosinophilic Disease (CURED) and Sunshine Charitable Foundation and its supporters, D. A. Bunning and D. G. Bunning.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A.M. is a consultant and/or international advisory board member for GSK, AstraZeneca, Sanofi, Oravax and Sartorious and is an inventor of patents owned by the Tel Aviv University. M.E.R. is a consultant for Pulm One, Spoon Guru, ClostraBio, Serpin Pharm, Allakos, Celldex Therapeutics, Nextstone One, Bristol Myers Squibb, AstraZeneca, Ellodi Pharma, GSK, Regeneron/Sanofi, Revolo Biotherapeutics and Guidepoint; has an equity interest in the first seven companies listed and royalties from reslizumab (Teva Pharmaceuticals), PEESSv2 (Mapi Research Trust) and UpToDate; and is an inventor of patents owned by Cincinnati Children’s Hospital. S.G.-T. declares no competing interests.
Peer review
Peer review information
Nature Immunology thanks Viktor Umansky and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Nick Bernard, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grisaru-Tal, S., Rothenberg, M.E. & Munitz, A. Eosinophil–lymphocyte interactions in the tumor microenvironment and cancer immunotherapy. Nat Immunol 23, 1309–1316 (2022). https://doi.org/10.1038/s41590-022-01291-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-022-01291-2
This article is cited by
-
Autotaxin–lysolipid signaling suppresses a CCL11–eosinophil axis to promote pancreatic cancer progression
Nature Cancer (2024)
-
Computational immunogenomic approaches to predict response to cancer immunotherapies
Nature Reviews Clinical Oncology (2024)
-
ATX restricts anti-tumor eosinophil responses
Nature Cancer (2024)
-
Multi-omics Analysis Identifies Hypoxia Subtypes and S100A2 as an Immunosuppressive Factor in Cervical Cancer
Reproductive Sciences (2024)
-
Immunophenotyping of peripheral blood in NSCLC patients discriminates responders to immune checkpoint inhibitors
Journal of Cancer Research and Clinical Oncology (2024)