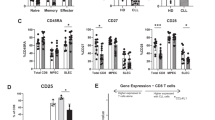
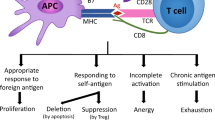
Abstract
Exhausted CD8 T cells (TEX) are a distinct state of T cell differentiation associated with failure to clear chronic viruses and cancer. Immunotherapies such as PD-1 blockade can reinvigorate TEX cells, but reinvigoration is not durable. A major unanswered question is whether TEX cells differentiate into functional durable memory T cells (TMEM) upon antigen clearance. Here, using a mouse model, we found that upon eliminating chronic antigenic stimulation, TEX cells partially (re)acquire phenotypic and transcriptional features of TMEM cells. These ‘recovering’ TEX cells originated from the T cell factor (TCF-1+) TEX progenitor subset. Nevertheless, the recall capacity of these recovering TEX cells remained compromised as compared to TMEM cells. Chromatin-accessibility profiling revealed a failure to recover core memory epigenetic circuits and maintenance of a largely exhausted open chromatin landscape. Thus, despite some phenotypic and transcriptional recovery upon antigen clearance, exhaustion leaves durable epigenetic scars constraining future immune responses. These results support epigenetic remodeling interventions for TEX cell–targeted immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated during this study are available within the paper. All sequencing data from this study are deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) and are accessible through a GEO Series accession code GSE150370. Any other relevant data are available from the corresponding author on request. Source data are provided with this paper.
Change history
29 September 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41590-021-01057-2
References
Kaech, S. M. & Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761 (2012).
McLane, L. M., Abdel-Hakeem, M. S. & Wherry, E. J. CD8 T cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol. 37, 457–495 (2019).
Wherry, E. J., Blattman, J. N., Murali-Krishna, K., van der Most, R. & Ahmed, R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77, 4911–4927 (2003).
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Fuller, M. J. & Zajac, A. J. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 170, 477–486 (2003).
Wherry, E. J., Barber, D. L., Kaech, S. M., Blattman, J. N. & Ahmed, R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl Acad. Sci. USA 101, 16004–16009 (2004).
Shin, H., Blackburn, S. D., Blattman, J. N. & Wherry, E. J. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 204, 941–949 (2007).
Tan, J. T. et al. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 195, 1523–1532 (2002).
Doering, T. A. et al. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity 37, 1130–1144 (2012).
Crawford, A. et al. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity 40, 289–302 (2014).
Pauken, K. E. et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354, 1160–1165 (2016).
Sen, D. R. et al. The epigenetic landscape of T cell exhaustion. Science 354, 1165–1169 (2016).
Philip, M. et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 545, 452–456 (2017).
Mognol, G. P. et al. Exhaustion-associated regulatory regions in CD8+ tumor-infiltrating T cells. Proc. Natl Acad. Sci. USA 114, E2776–E2785 (2017).
Youngblood, B. et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8+ T cells. Immunity 35, 400–412 (2011).
Ghoneim, H. E. et al. De novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell 170, 142–157.e19 (2017).
Khan, O. et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature 571, 211–218 (2019).
Alfei, F. et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 571, 265–269 (2019).
Scott, A. C. et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature 571, 270–274 (2019).
Yao, C. et al. Single-cell RNA-seq reveals TOX as a key regulator of CD8+ T cell persistence in chronic infection. Nat. Immunol. 20, 890–901 (2019).
Seo, H. et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion. Proc. Natl Acad. Sci. USA 116, 12410–12415 (2019).
Wang, X. et al. TOX promotes the exhaustion of antitumor CD8+ T cells by preventing PD1 degradation in hepatocellular carcinoma. J. Hepatol. 71, 731–741 (2019).
Blackburn, S. D., Shin, H., Freeman, G. J. & Wherry, E. J. Selective expansion of a subset of exhausted CD8 T cells by αPD-L1 blockade. Proc. Natl Acad. Sci. USA 105, 15016–15021 (2008).
Paley, M. A. et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338, 1220–1225 (2012).
Utzschneider, D. T. et al. T cell factor 1-expressing memory-like CD8+ T cells sustain the immune response to chronic viral infections. Immunity 45, 415–427 (2016).
Im, S. J. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
Wu, T. et al. The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci. Immunol. 1, eaai8593 (2016).
He, R. et al. Follicular CXCR5-expressing CD8+ T cells curtail chronic viral infection. Nature 537, 412–428 (2016).
Beltra, J. C. et al. Developmental relationships of four exhausted CD8+ T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity 52, 825–841.e8 (2020).
Hudson, W. H. et al. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1+ stem-like CD8+ T cells during chronic infection. Immunity 51, 1043–1058.e4 (2019).
Zander, R. et al. CD4+ T cell help is required for the formation of a cytolytic CD8+ T cell subset that protects against chronic infection and cancer. Immunity 51, 1028–1042.e4 (2019).
Jadhav, R. R. et al. Epigenetic signature of PD-1+ TCF1+ CD8 T cells that act as resource cells during chronic viral infection and respond to PD-1 blockade. Proc. Natl Acad. Sci. USA 116, 14113–14118 (2019).
Angelosanto, J. M., Blackburn, S. D., Crawford, A. & Wherry, E. J. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J. Virol. 86, 8161–8170 (2012).
Utzschneider, D. T. et al. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat. Immunol. 14, 603–610 (2013).
Martin, B. et al. Restoration of HCV-specific CD8+ T cell function by interferon-free therapy. J. Hepatol. 61, 538–543 (2014).
Burchill, M. A., Golden-Mason, L., Wind-Rotolo, M. & Rosen, H. R. Memory re-differentiation and reduced lymphocyte activation in chronic HCV-infected patients receiving direct-acting antivirals. J. Viral Hepat. 22, 983–991 (2015).
Wieland, D. et al. TCF1+ hepatitis C virus-specific CD8+ T cells are maintained after cessation of chronic antigen stimulation. Nat. Commun. 8, 15050 (2017).
Feld, J. J. et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N. Engl. J. Med. 373, 2599–2607 (2015).
Robert, C. et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 20, 1239–1251 (2019).
Larkin, J. et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 381, 1535–1546 (2019).
Pircher, H. et al. Molecular analysis of the antigen receptor of virus-specific cytotoxic T cells and identification of a new V alpha family. Eur. J. Immunol. 17, 1843–1846 (1987).
Odorizzi, P. M., Pauken, K. E., Paley, M. A., Sharpe, A. & Wherry, E. J. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J. Exp. Med. 212, 1125–1137 (2015).
Blattman, J. N. et al. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J. Exp. Med. 195, 657–664 (2002).
Qiu, X. et al. Single-cell mRNA quantification and differential analysis with Census. Nat. Methods 14, 309–315 (2017).
Miller, B. C. et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Callendret, B. et al. T-cell immunity and hepatitis C virus reinfection after cure of chronic hepatitis C with an interferon-free antiviral regimen in a chimpanzee. Hepatology 60, 1531–1540 (2014).
Tonnerre, P. et al. Differentiation of exhausted CD8+ T cells after termination of chronic antigen stimulation stops short of achieving functional T cell memory. Nat. Immunol. https://doi.org/10.1038/s41590-021-00982-6 (2021).
Sen, D. R. et al. Epigenetic scars of CD8+ T cell exhaustion persist after cure of chronic infection in humans. Nat. Immunol. https://doi.org/10.1038/s41590-021-00979-1 (2021).
Hensel, N. et al. Memory-like HCV-specific CD8+ T cells retain a molecular scar after cure of chronic HCV infection. Nat. Immunol. 22, 229–239 (2021).
Butler, A. et al. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Chen, Y. C. et al. IKAP—identifying K mAjor cell Population groups in single-cell RNA-sequencing analysis. Gigascience 8, giz121 (2019).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Hahne, F. & Ivanec, R. in Statistical Genomics: Methods and Protocols (eds Mathe, E. & Davis, S.) 335–351 (Humana Press, 2016).
McLean, C. Y. et al. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 28, 495–501 (2010).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Zhang, K., Wang, M., Zhao, Y. & Wang, W. Taiji: system-level identification of key transcription factors reveals transcriptional waves in mouse embryonic development. Sci. Adv. 5, eaav3262 (2019).
Acknowledgements
We thank members of the Wherry laboratory for insightful comments, critical reading of the manuscript and advice. This work was supported by NIH grants AI155577, AI105343, AI115712, AI117950, AI108545, AI082630 and CA210944 (to E.J.W.). E.J.W. is supported by the Parker Institute for Cancer Immunotherapy, which supports the cancer immunology program at UPenn. M.S.A.-H. is a Cancer Research Institute (CRI) Irvington Postdoctoral Fellow and is supported by Fonds de Recherche Québec–Santé (FRQS) and Canadian Network on Hepatitis C (CanHepC) Postdoctoral Fellowships. CanHepC is funded by a joint initiative from CIHR (NHC-142832) and the Public Health Agency of Canada. All diagrams created with BioRender.com.
Author information
Authors and Affiliations
Contributions
M.S.A.-H. and E.J.W. conceived and designed the experiments. M.S.A.-H. performed the experiments with help from J.-C.B., E.S., M.-A.A., K.N. and D.M. M.S.A.-H. analyzed all flow cytometry experiments. S.M. and M.S.A.-H. analyzed scRNA-seq and ATAC-seq data. Z.C. and J.L.J. helped with scRNA-seq and ATAC-seq experiments, respectively. J.R.G. provided scripts and helped perform quality control analysis of ATAC-seq samples. G.V. provided critical comments for ATAC-seq analysis. M.S.A.-H., A.R.G. and E.J.W. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
E.J.W. is a member of the Parker Institute for Cancer Immunotherapy. E.J.W. has consulting agreements with and/or is on the scientific advisory board for Merck, Marengo Therapeutics, Janssen, Related Sciences, Synthekine and Surface Oncology. E.J.W. is a founder of Surface Oncology and Arsenal Biosciences. E.J.W. has a patent licensing agreement on the PD-1 pathway with Roche/Genentech. The remaining authors declare no competing interests.
Additional information
Peer review information Nature Immunology thanks Enrico Lugli, Mary Philip and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. L. A. Dempsey was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Upon antigen elimination TEX downregulate several exhaustion markers and upregulate some TMEM features.
a, General gating strategy; dot plots in all figures are for transferred donor P14 cells gated on live CD8 + congenic cells. For surface stains, cells were also gated on Db-GP33 tetramer+ cells (yellow rectangle). b, Representative dot plots for the association between TCF-1 and Ly108 (Slamf6) expression on the day of adoptive transfer (d0), and d28 post-transfer. c, Left, representative dot plots of Ly108 and PD-1 expression. Right, percentages of different TEX subsets. N = 9 biologically independent experiments for d0 and n = 4 for d28. d, Left, representative histograms for CD127. Right, CD127 expression as ∆MFI, corresponding to Fig. 1c. e, Left, representative histograms for Eomes. Right, Eomes expression as ∆MFI, corresponding to Fig. 1e. Data presented in (c-e) as mean + /- SD. f, Representative dot plots of cytokine production (IFNγ) and LAMP-1 (CD107a) expression on unstimulated splenocytes, corresponding to Fig. 1g. g, Left, experimental model, stability of TEX P14 phenotype post-transfer was examined at day >180 post-transfer (d193-d570) into infection-free mice. Top row, representative dot plots of CD127 and PD-1, CD127 and CD62L, and TCF-1 and Tim-3 expression. To the right of dot plots, percentages of the different TEX subsets, either compared to compared to TMEM P14 cells from mice that cleared LCMV-Arm (d67-d238 p.i.). Bottom, representative histograms of Tox expression. To the right of histograms, levels of Tox expression. N = 2 biologically independent experiments, 2-5 REC-TEX mice/experiment. Data presented as mean + /- SD. All analyses for Extended Data Fig. 1 performed by unpaired two-tailed Student’s t-tests (Mann-Whitney test). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Extended Data Fig. 2 REC-TEX display an intermediate phenotype between TEX and TMEM in the P14 co-transfer or DbGP33 tetramer + non-P14 transfer approaches.
a, Experimental design for co-transfer of TEX and TMEM. b, Left, dot plots showing percentages of co-transferred TEX and TMEM P14 cells on d0 and d28 post-transfer. Right, fold change in number of donor TEX and TMEM P14 cells on d28 post-transfer relative to the original number adoptively transferred on d0 (estimating 10% take). N = 3 biologically independent experiments, 2-4 mice/experiment. c, Number of TEX and TMEM P14 cells per million CD8 T cells from peripheral blood mononuclear cells (PBMCs) longitudinally as a percentage of the number of cells calculated at baseline (d2.5 post-transfer). N = 1 experiment, 4-7 mice/time-point. Data presented as mean + /- S.E.M. Comparisons versus d2.5 analyzed by unpaired two-tailed Student’s t-tests (Mann-Whitney test). d, Left, representative dot plots of CD127 and PD-1, and TCF-1 and Tim-3 expression. Right, percentages of different subsets. e, Left panels, representative histograms for expression of indicated markers. Right, plots of ∆MFI. Data for (d-e) for PD-1 and CD127 n = 3 independent experiments, and for Tox and TCF-1 n = 1. Analyses for (b, d) paired two-tailed t-test (Wilcoxon matched-pairs signed rank test). *p < 0.05, **p < 0.01. f, Left, representative dot plots for TIGIT and PD-1 expression. Right, percentages of IRs co-expression on P14 cells. N = 1 experiment. g, Left, representative dot plots of IFNγ production and LAMP-1/CD107a expression on P14 cells. Left, percentages of polyfunctionality on P14 cells. N = 2 independent experiments. For (b-g) red and blue percentages are for TEX and TMEM P14 cells, respectively. Data for d28 from 2-4 mice per experiment. h, Experimental design for adoptive transfer of CD8 + T cells containing DbGP33-specific TEX or TMEM. i, Left, representative dot plots for CD127 and PD-1 expression on donor Db-GP33 specific T cells. Right, percentages of different subsets. j, Left, representative histograms for level of expression of PD-1 or CD127. Right, plots for level of expression of PD-1 and CD127. Data (i-j) n = 2 independent experiments, 3-8 mice/group/experiment for d28. Data presented as mean + /- SD. Analyses for (i-j) by unpaired two-tailed Student’s t-tests (Mann-Whitney test). ***p < 0.001, ****p < 0.0001.
Extended Data Fig. 3 REC-TEX are transcriptionally distinct from both TEX and TMEM.
a, Top, general gating strategy for sorting; sorted cells were gated on live CD8 + DbGP33-tetramer+ cells. Bottom, dot plots showing purity post-sorting for samples used for scRNAseq. b, tSNE plot of unsupervised clustering of combined TEX, TMEM, and REC-TEX cells. b, Contribution of TEX, TMEM, and REC-TEX to each cluster in (b). c, Heatmap of the top differentially expressed genes (DEGs) between TEX, TMEM, and REC-TEX. d, Violin plots for genes differentially expressed by REC-TEX compared to both TEX and TMEM. e, tSNE plots of TEX, TMEM, and REC-TEX cells with overlay of Pdcd1, Lag3, and Gzmb genes.
Extended Data Fig. 4 REC-TEX are transcriptionally heterogeneous and distinct from the TCF-1+ TEX subset.
a, Circos plots of different REC-TEX clusters compared to TEX and TMEM signatures. b, Violin plots for individual gene expression by the different REC-TEX clusters from Fig. 2c. c, Pseudotime plot of TEX and REC-TEX with overlay of Tox (d) Pseudotime plot of TEX and REC-TEX with overlay of upregulated gene signature in TCF-1– TEX subset from Utzschneider et al.25. e, Volcano plot showing selected differentially expressed genes between TCF-1+ TEX and REC-TEX.
Extended Data Fig. 5 Partial recovery from exhaustion in REC-TEX is associated with preferential survival of the progenitor TEX subset.
a, Left, representative dot plot of TCF-1 expression in Blimp1-YFP TEX P14 cells on d20 p.i. with LCMV-Cl13. Middle, dot plots showing sorting purity of Blimp1-YFP– (that is TCF-1+) progenitor TEX (Prog-TEX) and Blimp1-YFP+ (that is TCF-1–) terminal TEX (Term-TEX). Right, experimental design for co-transfer of Prog-TEX and Term-TEX into infection-free recipient mice. b, Left, representative dot plots for frequency of donor Blimp1-YFP– Prog-TEX or Blimp1-YFP+ Term-TEX P14 cells, on the day of adoptive transfer (d0) and d26 post-transfer. Middle, number of P14 cells for Prog-TEX and Term-TEX on d0 and d26 post-transfer (estimating 10% take on d0). Right, absolute number of P14 cells per spleen for each subset on d26 post-transfer. c, Left, representative dot plots of CD127 and PD-1 expression. Right, percentages of CD127+ or PD-1+CD127– for both subsets on d0 and d26 post-transfer, and absolute numbers per spleen of CD127 + cells for each subset on d26 post-transfer. d, Left, representative histograms for expression of CD127 or PD-1 on Prog-TEX and Term-TEX on d0 and d26 post-transfer, and on endogenous GP33-tetramer+ TMEM of recipient mice on d26 post-transfer. Right, plots for MFIs. Data from one experiment, 2 mice.
Extended Data Fig. 6 Secondary effectors derived from REC-TEX and TMEM are distinct phenotypically and functionally.
a, Representative dot plots showing sorting purity. b, Representative dot plot for the frequency of co-transferred donor REC-TEX and TMEM P14 cells, on d-1. c, Absolute number of TEX and REC-TEX P14 cells per spleen on d8 post challenge with LCMV-Arm. N = 1 experiment, 3 mice. d, Representative dot plots and plots for PD-1 and KLRG1 expression from different organs. Data representative of two independent experiments, 4-5 mice. e, Representative dot plots of cytokine production (IFNγ and TNF) and CD107a expression on unstimulated splenocytes. Corresponding to Fig. 4e. f, Cytotoxicity assay of secondary effector cells (d8 post LCMV-Arm challenge) from separately transferred REC-TEX or TMEM P14 cells into naïve mice. Left, plots of % specific killing for REC-TEX compared to TMEM. Middle and right, number of effector cells and ratio of effector:target cells in REC-TEX containing wells compared to TMEM wells. N = 1 experiment. Data presented as mean + /- SD. Unpaired two-tailed Student’s t-tests (Mann-Whitney test). g, Percentages of CD127+CD62L+ and Tox+PD-1+, and expression of PD-1, Tox and Eomes on co-transferred REC-TEX and TMEM P14s at d30 post-challenge with LCMV-Arm. N = 2 biologically independent experiments, 2-5 mice/experiment. h, Absolute number per spleen for cells derived from TMEM and REC-TEX P14 cells (left), endogenous CD8 + T cells (middle), and endogenous GP33-tetramer+ cells (right), on d8 post LCMV-Arm challenge. i, Left, representative dot plots of T-bet and Eomes expression. Right, percentages of Eomes+T-bet+. j, Left, representative dot plots for GzmB expression. Right, percentages of GzmB expressing cells. k, Left, histograms for expression of Tox and T-bet. Right, plots for levels of expression as ∆MFI (compared to naïve). l, Representative dot plots and plots for PD-1 and KLRG1 from different organs from anti-PD-L1 treated mice compared to control PBS-treated mice. Data for (h-l) N = 2 biologically independent experiments, 2-4 mice/group/experiment. All analyses for Extended Data Fig. 6, for co-transferred cells performed by paired two-tailed t-test (Wilcoxon matched-pairs signed rank test). PBS versus αPD-L1 (h-l) analyzed using unpaired two-tailed Student’s t-tests (Mann-Whitney test). *p < 0.05, **p < 0.01, ***p < 0.001.
Extended Data Fig. 7 REC-TEX remain epigenetically similar to TEX and bear signatures from both the TCF-1+ and TCF-1– TEX subsets.
a, Representative dot plots showing sorting purity for ATACseq samples. b, Correlation of normalized peak enrichment between replicates of for ATACseq samples for each cell type. R2 indicates degree of correlation between replicates at p < 2.2e-16, calculated by Spearman correlation. c, Pearson correlation of ATACseq profiles from TEX, TMEM, and REC-TEX samples. d, Principal component analysis (PCA) of ATACseq profiles for naïve, TEX, TMEM, and REC-TEX P14 cells. e, Venn diagram showing the overlap between OCRs significantly open in REC-TEX versus TEX (REC-TEXUP_vsTEX) (from Fig. 5b) and UP_TMEM _vsTEX. f, Representative ATACseq tracks at indicated loci from naïve, TEX, TMEM, and REC-TEX. g, PCA of ATACseq profiles for TEX, TMEM, and REC-TEX samples from our current dataset compared to TMEM, CXCR5– Tim-3+ TEX and CXCR5– Tim-3+ TEX from Jadhav et al.32 h, Heatmap showing chromatin accessibility of CXCR5– Tim-3+ TEX and CXCR5– Tim-3+ TEX from Jadhav et al.32 for the 182 differential peaks from REC-TEX vs. TEX signature of the current dataset (presented in Fig. 6b). i, Representative ATACseq tracks at indicated loci from TEX and REC-TEX samples from the current dataset compared to the four TEX subsets from Beltra et al.29.
Extended Data Fig. 8 Correlation between transcriptional and epigenetic profiles of REC-TEX identifies epigenetically fixed networks.
a, Genes from scRNAseq with significantly different expression between REC-TEX and TEX that are associated with more accessible OCRs in REC-TEX compared to both TEX and TMEM. b, Bubble plot showing significantly different biological processes associated with significantly different OCRs between REC-TEX and TMEM identified by GREAT. c, TFs with significantly enriched motifs in OCRs in REC-TEX versus TMEM (top half, purple circles) or in TMEM versus REC-TEX (bottom half, blue circles). Pairwise analysis using HOMER. –log10 p-value calculated by hypergeometric distribution test. d, Venn diagrams showing the overlap in TFBS between TEX, TMEM, and REC-TEX for some transcription factors from PageRank analysis (Fig. 6g), Tcf7, Hic1 and Hsf1. e, Heatmap of the 58 OCRs showing significantly differential accessibility in REC-TEX compared to TEX from the ∆Tox-TEX versus wild-type TEX signature by Khan et al.17.
Extended Data Fig. 9 Eliminating chronic antigen stimulation induces varying degrees of transcriptional and epigenetic recovery in TEX.
Upon eliminating chronic antigen stimulation, exhausted T cells (TEX) failed to fully differentiate into bona fide memory T cells (TMEM), but rather gave rise to cells with mixed exhaustion and memory features, recovering- TEX cells (REC-TEX). The degree of phenotypic and transcriptional recovery towards memory was not associated with a similar degree of recovery in recall capacity, mainly due to limited changes in the chromatin accessibility landscape. Ag, antigen; IL-7, interleukin-7; MPEC, memory precursor effector cells; SLEC, short-lived effector cells; and TEFF, effector T cells. Diagram created with Biorender.com.
Supplementary information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Rights and permissions
About this article
Cite this article
Abdel-Hakeem, M.S., Manne, S., Beltra, JC. et al. Epigenetic scarring of exhausted T cells hinders memory differentiation upon eliminating chronic antigenic stimulation. Nat Immunol 22, 1008–1019 (2021). https://doi.org/10.1038/s41590-021-00975-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-021-00975-5
This article is cited by
-
Gene editing technology to improve antitumor T-cell functions in adoptive immunotherapy
Inflammation and Regeneration (2024)
-
Type 1 interferons and Foxo1 down-regulation play a key role in age-related T-cell exhaustion in mice
Nature Communications (2024)
-
Liver in infections: a single-cell and spatial transcriptomics perspective
Journal of Biomedical Science (2023)
-
Synergetic regulation of cancer cells and exhausted T cells to fight cold tumors with a fluorinated EGCG-based nanocomplex
Journal of Nanobiotechnology (2023)
-
The inhibitory receptors PD1, Tim3, and A2aR are highly expressed during mesoCAR T cell manufacturing in advanced human epithelial ovarian cancer
Cancer Cell International (2023)