Abstract
Regulatory T (Treg) cells are a barrier for tumor immunity and a target for immunotherapy. Using single-cell transcriptomics, we found that CD4+ T cells infiltrating primary and metastatic colorectal cancer and non-small-cell lung cancer are highly enriched for two subsets of comparable size and suppressor function comprising forkhead box protein P3+ Treg and eomesodermin homolog (EOMES)+ type 1 regulatory T (Tr1)-like cells also expressing granzyme K and chitinase-3-like protein 2. EOMES+ Tr1-like cells, but not Treg cells, were clonally related to effector T cells and were clonally expanded in primary and metastatic tumors, which is consistent with their proliferation and differentiation in situ. Using chitinase-3-like protein 2 as a subset signature, we found that the EOMES+ Tr1-like subset correlates with disease progression but is also associated with response to programmed cell death protein 1–targeted immunotherapy. Collectively, these findings highlight the heterogeneity of Treg cells that accumulate in primary tumors and metastases and identify a new prospective target for cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Single-cell RNA-seq and TCR sequencing data that support the findings of this study have been deposited in the ArrayExpress archive under accession no. E-MTAB-7006. Gene lists derived from differential expression analysis and refined GSEA gene lists are available in the supplementary table files. All data generated during the current study are available from the corresponding authors upon request. Source data are provided with this paper.
References
Dobrzanski, M. J. Expanding roles for CD4 T cells and their subpopulations in tumor immunity and therapy. Front. Oncol. 3, 63 (2013).
Shang, B., Liu, Y., Jiang, S.-J. & Liu, Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci. Rep. 5, 15179 (2015).
Burzyn, D. et al. A special population of regulatory T cells potentiates muscle repair. Cell 155, 1282–1295 (2013).
Cipolletta, D. et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 486, 549–553 (2012).
Sefik, E. et al. Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science 349, 993–997 (2015).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Tanaka, A. & Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 27, 109–118 (2017).
Häringer, B., Lozza, L., Steckel, B. & Geginat, J. Identification and characterization of IL-10/IFN-γ-producing effector-like T cells with regulatory function in human blood. J. Exp. Med. 206, 1009–1017 (2009).
Pot, C. et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J. Immunol. 183, 797–801 (2009).
Zhang, P. et al. Eomesodermin promotes the development of type 1 regulatory T (TR1) cells. Sci. Immunol. 2, eaah7152 (2017).
Gagliani, N. et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med. 19, 739–746 (2013).
Gruarin, P. et al. Eomesodermin controls a unique differentiation program in human IL-10 and IFN-γ coproducing regulatory T cells. Eur. J. Immunol. 49, 96–111 (2019).
Mascanfroni, I. D. et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat. Med. 21, 638–646 (2015).
Roncarolo, M. G., Gregori, S., Bacchetta, R., Battaglia, M. & Gagliani, N. The biology of T regulatory type 1 cells and their therapeutic application in immune-mediated diseases. Immunity 49, 1004–1019 (2018).
Dobrzanski, M. J. et al. Immunotherapy with IL-10- and IFN-γ-producing CD4 effector cells modulate ‘Natural’ and ‘Inducible’ CD4 TReg cell subpopulation levels: observations in four cases of patients with ovarian cancer. Cancer Immunol. Immunother. 61, 839–854 (2012).
Marshall, N. A. et al. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood 103, 1755–1762 (2004).
Pedroza-Gonzalez, A. et al. Tumor-infiltrating plasmacytoid dendritic cells promote immunosuppression by Tr1 cells in human liver tumors. Oncoimmunology 4, e1008355 (2015).
Fan, X. & Rudensky, A. Y. Hallmarks of tissue-resident lymphocytes. Cell 164, 1198–1211 (2016).
Stubbington, M. J. T., Rozenblatt-Rosen, O., Regev, A. & Teichmann, S. A. Single-cell transcriptomics to explore the immune system in health and disease. Science 358, 58–63 (2017).
Azizi, E. et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell 174, 1293–1308.e36 (2018).
Guo, X. et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat. Med. 24, 978–985 (2018).
Tirosh, I. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016).
Zheng, C. et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell 169, 1342–1356.e16 (2017).
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015).
Webber, W. M. A., & Zobel, J. A similarity measure for indefinite rankings. ACM Trans. Inf. Syst. 28, 20 (2010).
De Simone, M. et al. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory cells. Immunity 45, 1135–1147 (2016).
Plitas, G. et al. Regulatory T cells exhibit distinct features in human breast cancer. Immunity 45, 1122–1134 (2016).
Togashi, Y., Shitara, K. & Nishikawa, H. Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat. Rev. Clin. Oncol. 16, 356–371 (2019).
Alfen, J. S. et al. Intestinal IFN-γ-producing type 1 regulatory T cells coexpress CCR5 and programmed cell death protein 1 and downregulate IL-10 in the inflamed guts of patients with inflammatory bowel disease. J. Allergy Clin. Immunol. 142, 1537–1547.e8 (2018).
Facciotti, F. et al. Evidence for a pathogenic role of extrafollicular, IL-10-producing CCR6+B helper T cells in systemic lupus erythematosus. Proc. Natl Acad. Sci. USA 117, 7305–7316 (2020).
Zhang, L. et al. Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature 564, 268–272 (2018).
Keller, M. D. et al. T-cell receptor sequencing demonstrates persistence of virus-specific T cells after antiviral immunotherapy. Br. J. Haematol. 187, 206–218 (2019).
Areshkov, P. O., Avdieiev, S. S., Balynska, O. V., Leroith, D. & Kavsan, V. M. Two closely related human members of chitinase-like family, CHI3L1 and CHI3L2, activate ERK1/2 in 293 and U373 cells but have the different influence on cell proliferation. Int. J. Biol. Sci. 8, 39–48 (2012).
Lee, H.-O. et al. Lineage-dependent gene expression programs influence the immune landscape of colorectal cancer. Nat. Genet. 52, 594–603 (2020).
Sato, M. et al. Human lung epithelial cells progressed to malignancy through specific oncogenic manipulations. Mol. Cancer Res. 11, 638–650 (2013).
Smith, J. J. et al. Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer. Gastroenterology 138, 958–968 (2010).
Petitprez, F., Meylan, M., de Reyniès, A., Sautès-Fridman, C. & Fridman, W. H. The tumor microenvironment in the response to immune checkpoint blockade therapies. Front. Immunol. 11, 784 (2020).
Liu, D. et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat. Med. 25, 1916–1927 (2019).
Roncarolo, M. G. et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 212, 28–50 (2006).
Del Prete, G. et al. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J. Immunol. 150, 353–360 (1993).
Zielinski, C. E. et al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 484, 514–518 (2012).
Gagliani, N. et al. TH17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523, 221–225 (2015).
Ali, N. et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell 169, 1119–1129.e11 (2017).
Bluestone, J. A., Mackay, C. R., O’Shea, J. J. & Stockinger, B. The functional plasticity of T cell subsets. Nat. Rev. Immunol. 9, 811–816 (2009).
Sakaguchi, S., Vignali, D. A. A., Rudensky, A. Y., Niec, R. E. & Waldmann, H. The plasticity and stability of regulatory T cells. Nat. Rev. Immunol. 13, 461–467 (2013).
Alvisi, G. et al. IRF4 instructs effector Treg differentiation and immune suppression in human cancer. J. Clin. Invest. 130, 3137–3150 (2020).
Brummelman, J. et al. High-dimensional single cell analysis identifies stem-like cytotoxic CD8+ T cells infiltrating human tumors. J. Exp. Med. 215, 2520–2535 (2018).
Galletti, G. et al. Two subsets of stem-like CD8+ memory T cell progenitors with distinct fate commitments in humans. Nat. Immunol. 21, 1552–1562 (2020).
Kzhyshkowska, J., Yin, S., Liu, T., Riabov, V. & Mitrofanova, I. Role of chitinase-like proteins in cancer. Biol. Chem. 397, 231–247 (2016).
Libreros, S. & Iragavarapu-Charyulu, V. YKL-40/CHI3L1 drives inflammation on the road of tumor progression. J. Leukoc. Biol. 98, 931–936 (2015).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
McCarthy, D. J., Campbell, K. R., Lun, A. T. L. & Wills, Q. F. Scater: pre-processing, quality control, normalization and visualization of single-cell RNA-seq data in R. Bioinformatics 33, 1179–1186 (2017).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2019).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Brummelman, J. et al. Development, application and computational analysis of high-dimensional fluorescent antibody panels for single-cell flow cytometry. Nat. Protoc. 14, 1946–1969 (2019).
Lugli, E., Zanon, V., Mavilio, D. & Roberto, A. FACS analysis of memory T lymphocytes. Methods Mol. Biol. 1514, 31–47 (2017).
Acknowledgements
We thank M. Fakiola for discussions and critical revision of the manuscript, M. Moro and M. Crosti for technical assistance with cell sorting at Istituto Nazionale Genetica Molecolare and F. Colombo (Humanitas Flow Cytometry Core) for help with the FACSymphony A5 instrument setup. This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC) grant no. IG2016-ID18575, AIRC grant no. IG2019-ID 23826 and Fondazione AIRC under 5 per mille 2019 - ID 22759 program to M. Pagani; Cancer Research UK Accelerator Award no. 22794, Fondazione AIRC under 5 per mille 2018 - ID 21147 to S.A.; 21091 program to S.A., A.B. and S.S.; AIRC grant no. IG2017 - ID 20676 to E.L.; AIRC grant no. IG2018-ID21923 to A.B.; AIRC grant nos. IG2015-ID17448 and IG2019-ID23581 to J.G.; and by an unrestricted grant of the Fondazione Romeo ed Enrica Invernizzi. J.B. is a recipient of the Fondo di Beneficenza Intesa San Paolo fellowship from AIRC. E.M.C.M. is a recipient of the 2017 Fondazione Umberto Veronesi postdoctoral fellowship. Purchase of the FACSymphony A5 (BD Biosciences) has been in part defrayed by a grant from the Italian Ministry of Health (agreement no. 82/2015).
Author information
Authors and Affiliations
Contributions
R.J.P.B., G.R., E.L., M.D.S., P.G. and J.B. designed and performed the main experiments, analyzed the data, performed the bioinformatics analyses and contributed to the preparation of the manuscript. L.D., M. Passaro, F.G., V.R., S.M. and E.M.C.M. contributed to setting up all the bioinformatics pipelines. C.D., S.O., C.G. and C.C. performed the immunofluorescence analyses. S.C., R. Bason, Y.S., E.C., R. Bosotti, G.D., M.L.S., M.M., V.B., M.L. and G.A. contributed to the tumor sample processing and analysis. A.S.-B., A.A., D.P., E.B., G.V., M.A., P.N., A.G., N.Z., E.O., A.P.C., N.M. and S.S. coordinated the clinical contributions and pathology analyses. S.B. and A.B. discussed the results, provided advice and commented on the manuscript. R.J.P.B., E.L., G.R., J.G., S.A. and M. Pagani wrote the manuscript. J.G., A.L., S.A. and M. Pagani designed the study and supervised the research. All authors discussed and interpreted the results.
Corresponding authors
Ethics declarations
Competing interests
M. Pagani and S.A. are members of the board of directors and stakeholders of CheckmAb. E.L. has a consulting agreement with Achilles Therapeutics. All other authors declare no competing interests.
Additional information
Peer review information Nature Immunology thanks Stefani Spranger and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. L. A. Dempsey was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
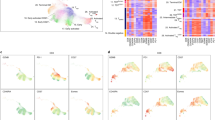
Extended Data Fig. 1 Overview of the experimental workflow.
a, Schematic overview of the experimental workflow. b, Single cell analysis quality controls. UMAP projection for both NSCLC and CRC as in Fig. 1b colored according to patient identity. Bar plots represent the percentage of cell deriving related to each patient for NSCLC and CRC clusters.
Extended Data Fig. 2 Single-cell analysis on additional NSCLC and CRC samples.
a, Upper panel: Unsupervised clustering of tumor infiltrating CD4+ T cells data generated with Chromium Single Cell 3’ protocol, UMAP projection is used for data visualization. Lower panel: Gene set enrichment analyses of reference CD4+ FOXP3+ and CD4+ EOMES+ gene sets (blue and red) presented as enrichment score profiles with the normalized enrichment score (NES) and nominal P-value. Expression and frequency of CD4+ FOXP3+ and EOMES+ subset gene signatures (blue and red) are shown across the different CD4+ cell clusters. b, UMAP projection for both NSCLC and CRC generated with Chromium Single Cell 3’ protocol colored according to patient identity. Bar plots the percentage of cell deriving related to each patient for NSCLC and CRC clusters.
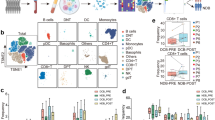
Extended Data Fig. 3 Characterization of EOMES+ Tr-1 like cells.
a, EOMES+ Tr1-like cells lack GNLY, CD25 and FOXP3 expression. One representative experiment out of all NSCLC (n= 48) and CRC (n= 28) patients is shown. b, Tr1-like cell sorting strategy: CCR5+CD27+Tr1-like cells were purified from the CD4+ fraction of CD127−CD25− Tr1-containing cells by cell sorting. c, Representative flow cytometry plots showing suppressive activity of EOMES+ Tr1-like cells vs FOXP3+ Treg cells isolated from tumor samples (NSCLC or CRC). Percentage of proliferating cells is indicated. d, EOMES+ Tr1-like cells and FOXP3+ regulatory T-cells suppress CD8+ T-cell proliferation. FACS- purified naïve CD8+ T-cells were labelled with CellTrace and stimulated with anti-CD3/28 beads in the presence of CD4+FOXP3+ (n=12) EOMES+ Tr1-like (n=11) subsets, naïve CD4+ T-cells (n=11) conventional effector cells: CD127-CD25-CCR5-CD4+ (n=12) and CCR6+CD127+CD25-CD4+ cells containing IL-10 producing helper T-cells (n=4). *** P<0.001 **P<0.01 (two-tailed unpaired t-test). Bars represent percentage of suppression ± s.e.m..Exact P values are provided in the Source Data.
Extended Data Fig. 4 TCR percentage and clonal size estimation.
a, Number of α and β chains combinations identified by TCR-sequencing for each patient. b, Number of cells and number of clones for different clonal size (grey=unique; yellow=2-4; orange=5-9; red>10 clones) for each NSCLC and CRC patient. c, Number of cells for each EOMES+ Tr1-like or FOXP3+ Treg cell clone for both CRC primary tumor (T) and their synchronous liver metastasis (M).
Extended Data Fig. 5 EOMES induction and Tr1-like cell stability.
Induction of EOMES in purified T-cell subsets upon stimulation with anti-CD3 antibodies and IL-12 or IL-27 in the absence and presence of CD28 co-stimulation. Results are presented as means of 6 biological replicates ± s.e.m. b, Induction of EOMES in CD4+ T memory cells upon stimulation with immobilized anti-CD3 antibodies, immature or mature CD1c+DC in the presence (black dots) or absence (white dots) of IL-4. Results are presented as average of ≥ 3 experiments ± s.e.m.. c, Expression of EOMES, GZMK and IL-10 in CD4+T-cell lines after 3 weeks of in vitro culture. T-cell subsets were sorted first ex vivo according to IL-7R and CD25 expression and then, after PMA/Ionomycin stimulation, according to IL-10 and CD40L expression with an IL-10 secretion assay. Bars represent means of n=2 biological replicates. d, Upper panel: EOMES, IL-10 and CD40L expression in a CD4 EOMES T-r1 clone at different time points: 3 weeks after generation (left) and after 6 (middle) or 12 months (right). Lower panel: Suppression of naïve CD4+ T-cell proliferation by the same CD4+EOMES+ Tr1 clone means of n=7 biological replicates is shown *P< 0.05 (two-tailed paired t-test). Exact P values are provided in the Source Data.
Extended Data Fig. 6 Multivariate Cox regression analysis on NSCLC, CRC and melanoma datasets.
6 a, Multivariate Cox regression analysis on NSCLC, CRC and melanoma datasets. P values were calculated by Chi-square Wald statistic b, Heatmap representing single-cell transcriptomic expression data for the indicated genes in melanoma patients c. Assessment of progression-free survival in a cohort of melanoma patients prior to a combined anti PD1/PD-L1 treatment divided by either low and high CD3 expression. Statistical significance was determined by two-tailed log-rank test.
Supplementary information
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Rights and permissions
About this article
Cite this article
Bonnal, R.J.P., Rossetti, G., Lugli, E. et al. Clonally expanded EOMES+ Tr1-like cells in primary and metastatic tumors are associated with disease progression. Nat Immunol 22, 735–745 (2021). https://doi.org/10.1038/s41590-021-00930-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-021-00930-4
This article is cited by
-
CTLs heterogeneity and plasticity: implications for cancer immunotherapy
Molecular Cancer (2024)
-
DISCERN: deep single-cell expression reconstruction for improved cell clustering and cell subtype and state detection
Genome Biology (2023)
-
Heterogeneity of circulating CD4+CD8+ double-positive T cells characterized by scRNA-seq analysis and trajectory inference
Scientific Reports (2022)
-
Metabolic reprogramming drives homeostasis and specialization of regulatory T cells in cancer
Oncology and Translational Medicine (2022)
-
Human T lymphocytes at tumor sites
Seminars in Immunopathology (2022)