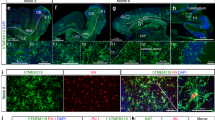
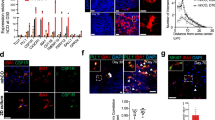
Abstract
Microglia and central nervous system (CNS)-associated macrophages (CAMs), such as perivascular and meningeal macrophages, are implicated in virtually all diseases of the CNS. However, little is known about their cell-type-specific roles in the absence of suitable tools that would allow for functional discrimination between the ontogenetically closely related microglia and CAMs. To develop a new microglia gene targeting model, we first applied massively parallel single-cell analyses to compare microglia and CAM signatures during homeostasis and disease and identified hexosaminidase subunit beta (Hexb) as a stably expressed microglia core gene, whereas other microglia core genes were substantially downregulated during pathologies. Next, we generated HexbtdTomato mice to stably monitor microglia behavior in vivo. Finally, the Hexb locus was employed for tamoxifen-inducible Cre-mediated gene manipulation in microglia and for fate mapping of microglia but not CAMs. In sum, we provide valuable new genetic tools to specifically study microglia functions in the CNS.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Raw data for new scRNA-seq or bulk RNA-seq have been deposited in the Gene Expression Omnibus, and are available at the following accession numbers: GSE148405 (scRNA-seq) and GSE148413 (bulk RNA-seq). All other data are published previously or are available from the corresponding authors on reasonable request.
Change history
11 August 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41590-020-0774-6
References
Colonna, M. & Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 35, 441–468 (2017).
Li, Q. & Barres, B. A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 18, 225–242 (2018).
Herz, J., Filiano, A. J., Smith, A., Yogev, N. & Kipnis, J. Myeloid cells in the central nervous system. Immunity 46, 943–956 (2017).
Priller, J. & Prinz, M. Targeting microglia in brain disorders. Science 365, 32–33 (2019).
Kierdorf, K., Masuda, T., Jordao, M. J. C. & Prinz, M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat. Rev. Neurosci. 20, 547–562 (2019).
Prinz, M., Erny, D. & Hagemeyer, N. Ontogeny and homeostasis of CNS myeloid cells. Nat. Immunol. 18, 385–392 (2017).
Prinz, M., Jung, S. & Priller, J. Microglia biology: one century of evolving concepts. Cell 179, 292–311 (2019).
Ueno, M. et al. Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci. 16, 543–551 (2013).
Hagemeyer, N. et al. Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. 134, 441–458 (2017).
Goldmann, T. et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 17, 797–805 (2016).
Van Hove, H. et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 22, 1021–1035 (2019).
Mrdjen, D. et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380–395 (2018).
Ajami, B. et al. Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat. Neurosci. 21, 541–551 (2018).
Bottcher, C. et al. Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat. Neurosci. 22, 78–90 (2019).
Jordao, M. J. C. et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363, eaat7554 (2019).
Masuda, T. et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 (2019).
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013).
Goldmann, T. et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 16, 1618–1626 (2013).
Zeisel, A. et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142 (2015).
Mildner, A. et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 10, 1544–1553 (2007).
Butovsky, O. et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 17, 131–143 (2014).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Hickman, S. E. et al. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 16, 1896–1905 (2013).
Hammond, T. R. et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50, 253–271 (2019).
Li, Q. et al. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron 101, 207–223 (2019).
Sango, K. et al. Mouse models of Tay-Sachs and Sandhoff diseases differ in neurologic phenotype and ganglioside metabolism. Nat. Genet. 11, 170–176 (1995).
Paolicelli, R. C. et al. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458 (2011).
Stevens, B. et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007).
Inoue, S., Inoue, M., Fujimura, S. & Nishinakamura, R. A mouse line expressing Sall1-driven inducible Cre recombinase in the kidney mesenchyme. Genesis 48, 207–212 (2010).
Buttgereit, A. et al. Sall1 is a transcriptional regulator defining microglia identity and function. Nat. Immunol. 17, 1397–1406 (2016).
Chappell-Maor, L. et al. Comparative analysis of CreER transgenic mice for the study of brain macrophages: a case study. Eur. J. Immunol. 50, 353–362 (2020).
Bruttger, J. et al. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity 43, 92–106 (2015).
Elmore, M. R. et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397 (2014).
Zhan, L. et al. Proximal recolonization by self-renewing microglia re-establishes microglial homeostasis in the adult mouse brain. PLoS Biol. 17, e3000134 (2019).
Huang, Y. et al. Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat. Neurosci. 21, 530–540 (2018).
Van Hove, H. et al. Identifying the variables that drive tamoxifen-independent CreERT2 recombination: implications for microglial fate mapping and gene deletions. Eur. J. Immunol. 50, 459–463 (2020).
Tay, T. L. et al. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci. 20, 793–803 (2017).
Fuger, P. et al. Microglia turnover with aging and in an Alzheimer’s model via long-term in vivo single-cell imaging. Nat. Neurosci. 20, 1371–1376 (2017).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Mildner, A., Huang, H., Radke, J., Stenzel, W. & Priller, J. P2Y12 receptor is expressed on human microglia under physiological conditions throughout development and is sensitive to neuroinflammatory diseases. Glia 65, 375–387 (2017).
Zrzavy, T. et al. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 140, 1900–1913 (2017).
Bennett, M. L. et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl Acad. Sci. USA 113, E1738–E1746 (2016).
Kaiser, T. & Feng, G. Tmem119-EGFP and Tmem119-CreERT2 transgenic mice for labeling and manipulating microglia. eNeuro. 6, ENEURO.0448-18.2019 (2019).
Wieghofer, P., Knobeloch, K. P. & Prinz, M. Genetic targeting of microglia. Glia 63, 1–22 (2015).
Dighe, A. S. et al. Tissue-specific targeting of cytokine unresponsiveness in transgenic mice. Immunity 3, 657–666 (1995).
Zehntner, S. P., Brisebois, M., Tran, E., Owens, T. & Fournier, S. Constitutive expression of a costimulatory ligand on antigen-presenting cells in the nervous system drives demyelinating disease. FASEB J. 17, 1910–1912 (2003).
Siao, C. J., Fernandez, S. R. & Tsirka, S. E. Cell type-specific roles for tissue plasminogen activator released by neurons or microglia after excitotoxic injury. J. Neurosci. 23, 3234–3242 (2003).
Rong, L. L. et al. RAGE modulates peripheral nerve regeneration via recruitment of both inflammatory and axonal outgrowth pathways. FASEB J. 18, 1818–1825 (2004).
Jung, S. et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 (2002).
Heppner, F. L. et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat. Med 11, 146–152 (2005).
Hashimshony, T. et al. CEL-Seq2: sensitive highly-multiplexed single-cell RNA-seq. Genome Biol. 17, 77 (2016).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 41, e108 (2013).
Herman, J. S., Sagar. & Grun, D. FateID infers cell fate bias in multipotent progenitors from single-cell RNA-seq data. Nat. Methods 15, 379–386 (2018).
Goldmann, T. et al. USP18 lack in microglia causes destructive interferonopathy of the mouse brain. EMBO J. 34, 1612–1629 (2015).
Raasch, J. et al. IκB kinase 2 determines oligodendrocyte loss by non-cell-autonomous activation of NF-κB in the central nervous system. Brain 134, 1184–1198 (2011).
Dann, A. et al. Cytosolic RIG-I-like helicases act as negative regulators of sterile inflammation in the CNS. Nat. Neurosci. 15, 98–106 (2012).
Prinz, M. et al. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity 28, 675–686 (2008).
Acknowledgements
We thank E. Barleon for excellent technical assistance and the Center for Animal Resource and Development (CARD, Kumamoto University) for providing research material. T. Masuda was supported by the KANAE Foundation for the Promotion of Medical Science and the Japan Society for the Promotion of Science (JSPS) as the JSPS Postdoctoral Fellow for Research Abroad. M.P. was supported by the Sobek Foundation, the Ernst-Jung Foundation, the German Research Foundation (DFG) (grant nos. SFB 992, SFB1160, SFB/TRR167, Reinhart-Koselleck-Grant and the Gottfried Wilhelm Leibniz-Prize) and the Ministry of Science, Research and Arts, Baden-Wuerttemberg (Sonderlinie ‘Neuroinflammation’). This study was supported by the DFG under Germany’s Excellence Strategy (grant no. CIBSS—EXC-2189, Project ID390939984). J.P. was supported by the DFG (grant nos. SFB/TRR167 B05 and B07), and the UK DRI Momentum Award. C.B. was supported by the DFG (grant no. SFB/TRR167 B05).
Author information
Authors and Affiliations
Contributions
T. Masuda, L.A., P.E., M.L., N.S., T. Misgeld, R.S., O.S., M.J.C.J., C.B., K.K., D.G., A.V. and K.P.K. conducted experiments and analyzed the data. M.P., K.P.K., S.J., M.M.L. and J.P. analyzed the data, contributed to the in vivo studies and provided mice or reagents. T. Masuda and M.P. supervised the project and wrote the manuscript, J.P. edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Editor recognition statement Zoltan Fehervari was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–14.
Supplementary Tables
Supplementary Tables 1–13.
Rights and permissions
About this article
Cite this article
Masuda, T., Amann, L., Sankowski, R. et al. Novel Hexb-based tools for studying microglia in the CNS. Nat Immunol 21, 802–815 (2020). https://doi.org/10.1038/s41590-020-0707-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-020-0707-4
This article is cited by
-
Fascin-1 limits myosin activity in microglia to control mechanical characterization of the injured spinal cord
Journal of Neuroinflammation (2024)
-
Border-associated macrophages in the central nervous system
Journal of Neuroinflammation (2024)
-
Cranial irradiation disrupts homeostatic microglial dynamic behavior
Journal of Neuroinflammation (2024)
-
Endothelial cells and macrophages as allies in the healthy and diseased brain
Acta Neuropathologica (2024)
-
The niche matters: origin, function and fate of CNS-associated macrophages during health and disease
Acta Neuropathologica (2024)