Abstract
Plasma membranes of animal cells are enriched for cholesterol. Cholesterol-dependent cytolysins (CDCs) are pore-forming toxins secreted by bacteria that target membrane cholesterol for their effector function. Phagocytes are essential for clearance of CDC-producing bacteria; however, the mechanisms by which these cells evade the deleterious effects of CDCs are largely unknown. Here, we report that interferon (IFN) signals convey resistance to CDC-induced pores on macrophages and neutrophils. We traced IFN-mediated resistance to CDCs to the rapid modulation of a specific pool of cholesterol in the plasma membrane of macrophages without changes to total cholesterol levels. Resistance to CDC-induced pore formation requires the production of the oxysterol 25-hydroxycholesterol (25HC), inhibition of cholesterol synthesis and redistribution of cholesterol to an esterified cholesterol pool. Accordingly, blocking the ability of IFN to reprogram cholesterol metabolism abrogates cellular protection and renders mice more susceptible to CDC-induced tissue damage. These studies illuminate targeted regulation of membrane cholesterol content as a host defense strategy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All original data are available from the corresponding author upon request.
References
Lange, Y., Swaisgood, M. H., Ramos, B. V. & Steck, T. L. Plasma membranes contain half the phospholipid and 90% of the cholesterol and sphingomyelin in cultured human fibroblasts. J. Biol. Chem. 264, 3786–3793 (1989).
Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 9, 125–138 (2008).
van Meer, G., Voelker, D. R. & Feigenson, G. W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 (2008).
Luo, J., Yang, H. & Song, B. L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 21, 225–245 (2020).
Blanc, M. et al. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity 38, 106–118 (2013).
Reboldi, A. et al. 25-hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science 345, 679–684 (2014).
Dang, E. V., McDonald, J. G., Russell, D. W. & Cyster, J. G. Oxysterol restraint of cholesterol synthesis prevents AIM2 inflammasome activation. Cell 171, 1057–1071 (2017).
Araldi, E. et al. Lanosterol modulates TLR4-mediated innate immune responses in macrophages. Cell Rep. 19, 2743–2755 (2017).
Blanc, M. et al. Host defense against viral infection involves interferon-mediated down-regulation of sterol biosynthesis. PLoS Biol. 9, e1000598 (2011).
York, A. G. et al. Limiting cholesterol biosynthetic flux spontaneously engages type I IFN signaling. Cell 163, 1716–1729 (2015).
Shibata, N. & Glass, C. K. Regulation of macrophage function in inflammation and atherosclerosis. J. Lipid Res. 50 (Suppl.), S277–S281 (2009).
Tall, A. R. & Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 15, 104–116 (2015).
Bauman, D. R. et al. 25-hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc. Natl Acad. Sci. USA 106, 16764–16769 (2009).
Liu, S.-Y. et al. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity 38, 92–105 (2013).
Viard, M. et al. Role of cholesterol in human immunodeficiency virus type 1 envelope protein-mediated fusion with host cells. J. Virol. 76, 11584–11595 (2002).
Goluszko, P. & Nowicki, B. Membrane cholesterol: a crucial molecule affecting interactions of microbial pathogens with mammalian cells. Infect. Immun. 73, 7791–7796 (2005).
Rawat, S. S. et al. Modulation of entry of enveloped viruses by cholesterol and sphingolipids. Mol. Membr. Biol. 20, 243–254 (2003).
Mazzon, M. & Mercer, J. Lipid interactions during virus entry and infection. Cell. Microbiol. 16, 1493–1502 (2014).
Tweten, R. K. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 73, 6199–6209 (2005).
Tweten, R. K., Hotze, E. M. & Wade, K. R. The unique molecular choreography of giant pore formation by the cholesterol-dependent cytolysins of Gram-positive bacteria. Annu. Rev. Microbiol. 69, 323–340 (2015).
Gilbert, R. J. Inactivation and activity of cholesterol-dependent cytolysins: what structural studies tell us. Structure 13, 1097–1106 (2005).
Timmer, A. M. et al. Streptolysin O promotes group A Streptococcus immune evasion by accelerated macrophage apoptosis. J. Biol. Chem. 284, 862–871 (2009).
Bhattacharjee, P. & Keyel, P. A. Cholesterol-dependent cytolysins impair pro-inflammatory macrophage responses. Sci. Rep. 8, 6458 (2018).
Corrotte, M., Fernandes, M. C., Tam, C. & Andrews, N. W. Toxin pores endocytosed during plasma membrane repair traffic into the lumen of MVBs for degradation. Traffic 13, 483–494 (2012).
McNeil, P. L. & Kirchhausen, T. An emergency response team for membrane repair. Nat. Rev. Mol. Cell Biol. 6, 499–505 (2005).
Romero, M. et al. Intrinsic repair protects cells from pore-forming toxins by microvesicle shedding. Cell Death Differ. 24, 798–808 (2017).
Oishi, Y. et al. SREBP1 contributes to resolution of pro-inflammatory TLR4 signaling by reprogramming fatty acid metabolism. Cell Metab. 25, 412–427 (2017).
Stetson, D. B. & Medzhitov, R. Type I interferons in host defense. Immunity 25, 373–381 (2006).
McNab, F., Mayer-Barber, K., Sher, A., Wack, A. & O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87–103 (2015).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Woodward, J. J., Iavarone, A. T. & Portnoy, D. A. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328, 1703–1705 (2010).
Pandey, A. K. et al. NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog. 5, e1000500 (2009).
Gay, A., Rye, D. & Radhakrishnan, A. Switch-like responses of two cholesterol sensors do not require protein oligomerization in membranes. Biophys. J. 108, 1459–1469 (2015).
Chakrabarti, R. S. et al. Variability of cholesterol accessibility in human red blood cells measured using a bacterial cholesterol-binding toxin. Elife 6, e23355 (2017).
Infante, R. E. & Radhakrishnan, A. Continuous transport of a small fraction of plasma membrane cholesterol to endoplasmic reticulum regulates total cellular cholesterol. Elife 6, e25466 (2017).
Endapally, S., Infante, R. E. & Radhakrishnan, A. Monitoring and modulating intracellular cholesterol trafficking using ALOD4, a cholesterol-binding protein. Methods Mol. Biol. 1949, 153–163 (2019).
Maxfield, F. R. & Wustner, D. Analysis of cholesterol trafficking with fluorescent probes. Methods Cell Biol. 108, 367–393 (2012).
Endapally, S. et al. Molecular discrimination between two conformations of sphingomyelin in plasma membranes. Cell 176, 1040–1053 (2019).
He, C. et al. High-resolution imaging and quantification of plasma membrane cholesterol by NanoSIMS. Proc. Natl Acad. Sci. USA 114, 2000–2005 (2017).
He, C. et al. Macrophages release plasma membrane-derived particles rich in accessible cholesterol. Proc. Natl Acad. Sci. USA 115, E8499–E8508 (2018).
Kandutsch, A. A. & Chen, H. W. Regulation of sterol synthesis in cultured cells by oxygenated derivatives of cholesterol. J. Cell Physiol. 85, 415–424 (1975).
Goldstein, J. L., DeBose-Boyd, R. A. & Brown, M. S. Protein sensors for membrane sterols. Cell 124, 35–46 (2006).
Panousis, C. G. & Zuckerman, S. H. Regulation of cholesterol distribution in macrophage-derived foam cells by interferon-γ. J. Lipid Res. 41, 75–83 (2000).
Keyel, P. A., Tkacheva, O. A., Larregina, A. T. & Salter, R. D. Coordinate stimulation of macrophages by microparticles and TLR ligands induces foam cell formation. J. Immunol. 189, 4621–4629 (2012).
Collins, J. L. et al. Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J. Med. Chem. 45, 1963–1966 (2002).
Das, A., Brown, M. S., Anderson, D. D., Goldstein, J. L. & Radhakrishnan, A. Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. Elife 3, e02882 (2014).
Pike, L. J. Lipid rafts: bringing order to chaos. J. Lipid Res. 44, 655–667 (2003).
Das, D. K., Baker, M. G. & Venugopal, K. Risk factors, microbiological findings and outcomes of necrotizing fasciitis in New Zealand: a retrospective chart review. BMC Infect. Dis. 12, 348 (2012).
Arif, N., Yousfi, S. & Vinnard, C. Deaths from necrotizing fasciitis in the United States, 2003–2013. Epidemiol. Infect. 144, 1338–1344 (2016).
Timmins, J. M. et al. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 115, 1333–1342 (2005).
Sag, D., Cekic, C., Wu, R., Linden, J. & Hedrick, C. C. The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity. Nat. Commun. 6, 6354 (2015).
Divakaruni, A. S. et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc. Natl Acad. Sci. USA 110, 5422–5427 (2013).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959).
Williams, K. J. et al. An essential requirement for the SCAP/SREBP signaling axis to protect cancer cells from lipotoxicity. Cancer Res. 73, 2850–2862 (2013).
Argus, J. P. et al. Development and application of FASA, a model for quantifying fatty acid metabolism using stable isotope labeling. Cell Rep. 25, 2919–2934 (2018).
He, C., Fong, L. G., Young, S. G. & Jiang, H. NanoSIMS imaging: an approach for visualizing and quantifying lipids in cells and tissues. J. Investig. Med. 65, 669–672 (2017).
Acknowledgements
This research was supported by NIH grants AI093768 (to S.J.B.), HL146358 (to S.J.B. and P.T.), AR073940 (to P.O.S), and HL136543 (to E.J.T.). M.S.L. is supported by Ruth L. Kirschstein National Research Service Award AI007323. The research described was also supported by an NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI grant (UL1TR001881). We thank S. Young, T. Weston and R.S. Jung for help with protein purification. We thank T. Weston for NanoSIMS sample preparation and SEM imaging. We thank A. Divakaruni for guidance with PFO permeabilization assays. We thank A. Radhakrishnan for ALO-D4 and full-length ALO plasmids. We thank S. Young, A. Hoffmann, Y. Du, R. Sun, T.-T. Wu, J. F. Miller and M. Li for thoughtful discussions.
Author information
Authors and Affiliations
Contributions
S.J.B. conceived the study. Q.D.Z. led the design and execution of experiments. X.C. codesigned and performed all flow cytometry experiments and data analysis. V.L.B., W.Y.H. and J.J.M. contributed to flow cytometry experiments. J.J.M. and M.S.L. contributed to protein purification and staining experiments. Q.D.Z., M.S.L. and R.D. developed and performed live-cell imaging assays. C.H. performed NanoSIMS analysis and contributed to protein purification. J.J.M. and E.B.K. contributed to RNA analysis. W.Y.H. and A.G.Y. performed GC–MS analysis with help from Q.D.Z. and E.B.K. K.J.W. conducted lipidomic studies. X.X., A.F., P.T. and E.J.T. contributed to Abcg1, Abca1 and SCAP KO studies. A.E.D. analyzed gene expression data. A.-C.F., P.O.S., M.S.L. and S.T.S. conceptualized and developed the in vivo SLO challenge assay. W.Y.H. contributed to data visualization. S.J.B., Q.D.Z., W.Y.H., X.C. and P.T. contributed to construction of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Editor recognition statement L. A. Dempsey was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
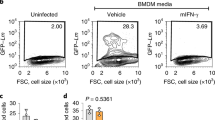
Extended Data Fig. 1 Interferon signaling mediates resistance to cholesterol-dependent cytolysins.
a, Percentage of PI-positive BMDMs treated with TLR1/2 agonist (Pam3CSK4; 50 ng/mL), TLR3 agonist (Poly(I:C); 1 μg/mL), TLR4 agonist (LPS; 50 ng/mL), TLR7 agonist (CL307; 100 nM), TLR9 (ODN1668; 100 nM) agonist, or unstimulated (NT) for 24 h and then challenged with PFO for up to 60 min in the presence of PI. Cells were imaged every 10 min to assess changes in PI incorporation. b, Percentage of PI-positive BMDMs treated with the various concentrations of TLR4 agonist for 24 h and then challenged with PFO for up to 60 min in the presence of PI. Cells were imaged every 7 min to assess changes in PI incorporation. c, Percentage of PI-positive BMDMs treated with IFN-β or IFN-γ (20 ng/mL) for 24 h and then challenged with PFO for 60 min in the presence of PI. d, Percentage of PI-positive BMDMs treated with NOD2 agonist (N-Glycolyl-MDP; 20 μg/ml), STING agonist (2’,3’cGAMP and c-di-GMP; both 2 μg/mL) for 24 h and then challenged with PFO for 60 min in the presence of PI. e, Percentage of PI-positive BMDMs treated with STING ligand for 24 h and then challenged with Streptolysin O (SLO) for 4 h in the presence of PI. f, Percentage of PI-positive BMDMs treated with IFN-β or IFN-γ (20 ng/mL) for 24 h and then challenged with SLO for 4 h in the presence of PI. g, Percentage of PI-positive BMDMs treated with IFN-β or IFN-γ (20 ng/mL) for 24 h and then challenged with ALO for 60 min in the presence of PI. h, Percentage of PI-positive BMDMs treated with IFN-β (100 ng/mL) or TLR1/2 agonist, or IFN-β (100 ng/mL) together with TLR1/2 agonist for 24 h and then challenged with PFO for 60 min in the presence of PI. i, Percentage of PI-positive BMDMs treated with IFN-β (100 ng/mL) or TLR1/2 agonist, or IFN-β (100 ng/mL) together with TLR1/2 agonist for 24 h and then challenged with SLO for 4 h in the presence of PI. Data are representatives of three independent experiments, and are shown as mean ± s.e.m. (n = 3). Statistical significance was determined using an RM one-way ANOVA with Dunnett’s correction (a-g) or a two-way ANOVA with Dunnett’s correction (h, i). ***P<0.001.
Extended Data Fig. 2 IFN signals decrease plasma membrane binding to ALO-D4 protein.
a, Confocal images of neutrophils stimulated with IFN-β or IFN-γ (20 ng/mL) for 6 h, and then stained with fluorescent ALO-D4 and DAPI. b, Violin plots of cellular fluorescent intensity quantified from a (n = 20334, 18546, 16290). c, Confocal images of WT or type I interferon receptor–deficient (IFNAR KO) BMDMs stimulated with TLR3 agonist (1 μg/mL) or IFN-β (20 ng/mL) for 24 h, and then stained with fluorescent ALO-D4 and DAPI. d, Violin plots of cellular fluorescent intensity quantified from c (n = 5543, 6682, 4673, 8231, 5201, 7906). Data are representatives of three independent experiments. Violin plots are shown with median (solid lines in b, d) and 25% and 75% percentiles (dashed lines in b), and statistical significance was determined using a Kruskal-Wallis test with Dunn’s correction. ***P<0.001. Scale bars in a, c represent 50 μm.
Extended Data Fig. 3 IFN signals reprogram cholesterol metabolism to decrease the pool of cholesterol targeted by CDCs.
a, Total cholesterol (nmol/107 cells) from C57BL/6 bone marrow–derived macrophages (BMDMs) stimulated with cGAMP (2 μg/mL), TLR3 agonist (Poly(I:C); 1 μg/mL), or unstimulated (NT) for 48 h. Total cholesterol was determined by GC-MS (n = 4). b, Total plasma membrane cholesterol (normalized to total FAMEs) from C57BL/6 bone marrow–derived macrophages (BMDMs) stimulated with IFN-γ (40 ng/mL) or unstimulated (NT) for 24 h (n = 4). c, Relative Fillipin III fluorescence intensity of plasma membranes of untreated macrophages or macrophages stimulated with IFN-β (20 ng/mL) or IFN-γ (20 ng/mL) for 24 h (n = 32, 38, 34, 42). MβCD-Cholesterol loaded macrophages indicate dynamic range of Fillipin III fluorescence and are included as a positive control. d, Confocal images of BMDM stimulated with IFN-β (20 ng/mL) for 24 h, and then stained with fluorescent ALO-D4 or OlyA and DAPI. Scale bar, 50 μm. e, Violin plots of cellular fluorescent intensity quantified from d (n = 2225, 2021, 2225, 2021). f, Cholera Toxin B staining of BMDM stimulated with IFN-β or IFN-γ (20 ng/mL) for 24 h. Median fluorescence intensity (MFI) are indicated on the left. Data are representative of three (a, d, e, f) independent experiments, three independent samples (c) or from 4 biological replicates (b). Data in a-c are shown as mean ± s.e.m., violin plots in e are shown with median (solid lines). Statistical significance was determined using an unpaired two-tailed Student’s t-test (a, b), a one-way ANOVA with Dunnett’s correction (c), or a two-tailed Mann–Whitney test (e) ***P<0.001.
Extended Data Fig. 4 Cholesterol synthesis is linked to CDC susceptibility.
a, Net synthesized cholesterol (nmol/107 cells) from C57BL/6 bone marrow–derived macrophages (BMDMs) stimulated with cGAMP (2 μg/mL), or unstimulated (NT) for 48 h. Synthesized cholesterol was determined by GC-MS and isotopomer spectral analysis modeling (n = 4). b, Net synthesized cholesterol (nmol/107 cells) from C57BL/6 bone marrow–derived macrophages (BMDMs) stimulated with TLR1/2 agonist (Pam3CSK4; 50 ng/mL), TLR3 agonist (Poly(I:C); 1 μg/mL), TLR4 agonist (LPS; 50 ng/mL), TLR7 agonist (CL307; 100 nM), TLR9 (ODN1668; 100 nM) agonist, or unstimulated (NT) for 48 h. Synthesized cholesterol was determined by GC-MS and isotopomer spectral analysis modeling (n = 4). c, Percentage of PI–positive WT BMDMs treated with Simvastatin (1 μM) for 4 h and then challenged with PFO for 60 min in the presence of PI (n = 3). Data are representative of three independent experiments and are shown as mean + s.e.m. Statistical significance was determined using an unpaired two-tailed Student’s t-test (a), a one-way ANOVA with Dunnett’s correction (b), or a paired two-tailed Student’s t-test (c). ***P<0.001.
Extended Data Fig. 5 Production of 25-hydroxycholesterol is required to maintain changes in plasma membrane cholesterol and mediates resistance to CDCs.
a, Percentage of PI–positive control or CH25H KO BMDMs stimulated with IFNs (20 ng/mL) for 24 h and then challenged with PFO for 60 minutes in the presence of PI. b, Percentage of PI–positive control or CH25H KO BMDMs stimulated with IFNs (20 ng/mL) for 24 h and then challenged with SLO for 2 h in the presence of PI. Data are representative of three independent experiments and are shown as mean + s.e.m. (n = 3) and statistical significance was determined using a two-way ANOVA with Dunnett’s correction. ***P<0.001.
Extended Data Fig. 6 Cholesterol esterification contributes to CDC resistance of macrophages.
a, Quantification (nmol/107 cells) of cholesterol ester species (16:0. 18:1, 20:4) in BMDMs stimulated with TLR3 agonist (1 μg/mL) in FBS or LPDS for 48 h. CE species pool sizes were determined by direct infusion MS. b, Percentage of PI–positive WT BMDMs treated with IFN-β, or IFN-γ (20 ng/mL), or in combination with ACATi 58-035 (4.3 μM) for 24 h and then challenged with PFO for 60 min in the presence of PI. c, Quantification (nmol/107 cells) of total cholesterol ester (CE) in control or CH25H KO BMDMs stimulated with IFN-β (20 ng/mL) or IFN-γ (20 ng/mL) for 48 h. CE pool sizes were determined by direct infusion mass spectrometry. d, Percentage of PI-positive CH25H KO BMDMs treated with ACATi 58-035 (4.3 μM) for 24 h and then challenged PFO for 60 min in the presence of PI. e, Violin plots of cellular fluorescent intensity quantified from control or ABCA1 KO or ABCG1 KO BMDMs stimulated with IFNs (20 ng/mL) for 24 h and then stained with fluorescent ALO-D4 and DAPI (n = 5943, 4126, 5727, 6914, 5740, 7898; n=7201, 7532, 7563, 7417). f, Percentage of PI-positive control or ABCA1 KO BMDMs treated with IFNs (20 ng/mL) for 24 h and then challenged PFO for 60 min in the presence of PI. g, Percentage of PI-positive control or CH25H KO BMDMs treated with LXR agonist GW3965 (1 μM) for 24 h and then challenged PFO for 60 min in the presence of PI. Data are representatives of two (a, c) or three (b, d, e, f, g) independent experiments. Data in a, b, c, d, f and g are shown as mean + s.e.m. (n = 3 in a, b, d, g; n = 4 in c). Violin plots in e are shown with median (solid lines) and 25% and 75% percentiles (dashed lines). Statistical significance was determined using an unpaired two-tailed Student’s t-test (a), a two-way ANOVA with Tukey’s correction (b, c, f, g), a paired two-tailed Student’s t-test (d), or a Kruskal–Wallis test with Dunn’s correction (e). ***P<0.001.
Extended Data Fig. 7 25HC mediates protection to CDC induced tissue damage.
a, Lesion images of control or CH25H KO mice challenged intradermally with SLO (8 kU/mouse) for 48 h. b, Lesion images of vehicle or 25HC pretreated mice challenged intradermally with ALO (20 nM) for 48 h.
Supplementary information
Supplementary Information
Supplementary Table 1.
Rights and permissions
About this article
Cite this article
Zhou, Q.D., Chi, X., Lee, M.S. et al. Interferon-mediated reprogramming of membrane cholesterol to evade bacterial toxins. Nat Immunol 21, 746–755 (2020). https://doi.org/10.1038/s41590-020-0695-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-020-0695-4
This article is cited by
-
IL-10 constrains sphingolipid metabolism to limit inflammation
Nature (2024)
-
Murine Alox8 versus the human ALOX15B ortholog: differences and similarities
Pflügers Archiv - European Journal of Physiology (2024)
-
Lipid in microglial biology — from material to mediator
Inflammation and Regeneration (2023)
-
Visualization of accessible cholesterol using a GRAM domain-based biosensor
Nature Communications (2023)
-
Glucocorticoids increase tissue cell protection against pore-forming toxins from pathogenic bacteria
Communications Biology (2023)