Abstract
Laboratory mice have provided invaluable insight into mammalian immune systems. Yet the immune phenotypes of mice bred and maintained in conventional laboratory conditions often differ from the immune phenotypes of wild mammals. Recent work to naturalize the environmental experience of inbred laboratory mice—to take them where the wild things are (to borrow a phrase from Maurice Sendak), via approaches such as construction of exposure histories, provision of fecal transplants or surrogate mothering by wild mice, and rewilding—is poised to expand understanding, complementing genetic and phylogenetic research on how natural selection has shaped mammalian immune systems while improving the translational potential of mouse research.
Similar content being viewed by others
Main
As we have seen vividly in 2020–2021, infectious diseases pose perennial challenges to human health around the globe. Preclinical animal models, usually starting with experiments on ‘the laboratory mouse’, enable researchers to reveal extraordinary details of how the immune systems of mammals respond to such challenges. Many immune mechanisms elucidated in lab mice also hold in people and inform the design of clinical therapies. Indeed, lab mice can arguably serve as models for both mammalian immune function in general and specific human diseases1. Mouse models have thus generated some triumphs in translational medicine, such as immune checkpoint inhibitors that translated to several successful cancer treatments following modest optimization2.
Yet many treatments conferring health to mice in the lab fail to translate to people in the real world. For example, would-be treatments for septic shock and lymphoma have proven problematic in their translation to people (for example, see refs. 3,4). This difference in responsiveness to treatment may arise from the fact that the immune profiles of lab mice, especially the low density of mature T cells, the paucity of neutrophils and the low lipopolysaccharide (LPS) sensitivity, are a poor match for human immune profiles5,6,7. If the immune cell distributions of lab mice are highly unusual for adult mammals, it is perhaps no surprise that lab mice often do not exhibit humanlike immune responses or predict which vaccines or clinical interventions will work in people.
The immune phenotype and functional capacity of an animal to respond to infection emerge from some combination of genetics, epigenetics and environment. The wide immunological divergence between lab and field populations of Mus musculus is thus only partly due to their genetic divergence8,9. I note that lab mice represent a relatively narrow and idiosyncratic slice of murine genetic diversity10,11. Taking that as a given, I will focus the remainder of this Perspective on environmental rather than genetic drivers of immune phenotype in lab mice. Recent studies have taken the approach of working with genetically uniform inbred mice but varying their environmental experiences. Such work has the potential to dissect the contributions of environment to immune phenotype and thereby improve mice as model mammals as well as models for human diseases. In this Perspective, I provide a field immunologist’s view of what can be gleaned from this body of work. I also advocate that, as infectious diseases such as COVID-19 continue to plague us and demand development of new vaccines and treatments, we urgently need more immunological research to go where the wild things are, that is, to reveal how immune systems function in more natural environments.
In a sense, this Perspective is a mouse-centric update on a 2013 Commentary from Maizels and Nussey12. In these pages, I draw upon the logic of their figure and text advocating for research across a spectrum of wildness to understand the causes of immunological variation within and among populations. I fully agree that research on a wide variety of species in a wide variety of environments—for example, domesticated and wild animals, as well as diverse human populations—is essential to explaining immunological variation12, as recently reinforced by Flies and colleagues13. Indeed, I myself work on immune systems in species well beyond M. musculus. But for this piece, I explicitly focus on a primary research tool of biomedical immunology: the lab mouse. I begin by considering wildness as a multidimensional environmental state, before analyzing the pros and cons of a series of experimental studies putting lab mice into more realistic environments, to naturalize their immune systems.
What does it mean, environmentally and immunologically, to be wild?
Animals, including human beings, navigate complex environments that modify their physiology in myriad ways. The accrued antigenic experience of wild animals probably leaps first to mind as a modifier of immune function. Indeed, in comparison to the lab, natural environments will increase the likelihood of exposure to microbes, whether pathogenic or not, as well as parasites, allergens and diverse foods. These exposures activate immune cells, accelerate their developmental programs and diversify their specificities14,15. Wild animals are therefore likely to have more mature leukocytes and more diverse immune repertoires than lab animals, even beyond any genetic effects.
But there are other axes of wildness that might alter immune function, too. Many of these environment–immunity connections have recently been dissected mechanistically in lab animals. For example, seasonal and circadian rhythms interact to determine the magnitude of delayed-type hypersensitivity and endotoxin responses in lab-reared hamsters16, and exercise catalyzes systemic immunological and metabolic shifts driven by the type 2 cytokine interleukin (IL)-13 in lab mice17; a variety of additional examples are outlined below. Furthermore, emerging evidence of tight immunoneuroendocrine connections across the entire body (for example, see ref. 18), including in lab mice19 and wild mice20, suggests that the complex behavioral requirements of living wild (for example, to navigate or maintain vigilance for predators) may synergize systemically to alter the immune function of mammals. Because wild animals experience seasonal variation in the environment and exert their muscles and brains in search of food, protection and mates, we must therefore expect immunological impacts of wildness that go well beyond the accrual of antigenic experience.
The impacts of these combined, interacting environmental experiences are likely to be profound and to generate wide immunophenotypic divergence among wild animals. Even identical human twins exhibit wide differences in immune phenotype21, and, more generally, much of the immunological divergence among individual people is driven by environmental experience rather than heritable factors22,23. Environmental drivers of phenotypic diversification are relevant across biology: heterogeneity emerging via accumulated environmental experience underlies plasticity of behavior and brain structure among genetically identical mice24 and is arguably prime among the causes of individuality of organisms in ecological systems25.
So where does that leave us, if we aim to develop lab mouse models of mammalian immunology in general or of particular human infectious or inflammatory diseases? An array of recent approaches strike differing balances between realism and reductionism as we naturalize the lab mouse. Reductionism is arguably the best approach for specifying the mechanisms of changed immune function under controlled manipulation of one environmental variable at a time, whereas realism is the best approach for studying additive and interactive effects of disparate environmental variables on immune function and for testing whether discoveries made during reductionist experiments are robust to greater environmental complexity. Examples and pros and cons of each type of approach are discussed in the following sections, with reductionist dissection of abiotic and biotic environmental factors treated separately. This body of work is beginning to reveal multiple, nuanced environmental drivers of immunological wildness.
Bringing the wild indoors: abiotic factors
A common reductionist approach to dissecting complex environmental drivers of immune phenotype entails experimental manipulation of abiotic aspects of the real world that affect the immune system. Lab rodents of inbred, select genotypes and standardized prior environmental experience can be separated into groups that experience abiotic environmental conditions that match (in the control, conventional group) or diverge from (in the experimental, naturalized group) the usual conditions of research animal husbandry.
For example, most mouse houses are maintained at a temperature that is comfortable for clothed human experimenters but below the thermal optimum for such small animals as mice, especially those weighing less than 25 g (ref. 26). For mice of the C57BL/6 strain, a workhorse of immunology research, the thermoneutral zone has been estimated to begin at temperatures as high as 29–31 °C (ref. 27). Mice under standard husbandry conditions of ~21 °C therefore display energy expenditure greater than three times their basal metabolism28. Experiments in which control groups at 21 °C are compared to groups maintained at 30 °C show profound effects of thermal environment on physiology29, including immune function: for example, thermoneutral mice mount fever (rather than hypothermic) responses to LPS injection and exhibit enhanced antitumor activity of CD8+ T cells30. More recent work using a range of temperatures has suggested that 25.5–27.6 °C may be where lab mice best approximate the physiology of thermally comfortable human hosts; the authors advocate study of lab mice at a variety of temperatures to best match the diversity of human thermal regimes31. Impacts of environmental temperature on immune function are arguably unsurprising, given that thermogenesis and thus both fever32 and the maintenance of body temperature33 entail interleukins. Potential feedbacks between organismal and cellular immunometabolism34,35 would likely compound the effects of environmental temperature on the immune system.
Another important impact of abiotic environment on immune function is via the photoperiod. Independently of temperature, light–dark cycles alter the type and magnitude of immune responses in lab mice, because trafficking36 and function37 of lymphocytes and dendritic cells vary rhythmically across the circadian cycle. These cycles presumably alter the frequency of cell–cell interactions and, thereby, the speed and efficiency of induced responses38. Recent evidence that the interaction of circadian and seasonal rhythms shapes peripheral blood leucocyte concentrations and endotoxin responses16 suggests that it may not model human immunology well to keep all laboratory mice in constant 12-h light/12-h dark cycles.
Indeed, these results raise important questions for animal husbandry. Are mice in lab facilities thermally stressed and thus immunologically biased, and, if so, should we ‘warm the mouse’ to improve models (for example, for tumor immunology30)? Should light–dark cycles and temperatures in the lab really be for the comfort and convenience of the researchers, if we want to improve mouse models for mammalian immunology in general, as well as models of human diseases? What aspects of mammalian life are we modeling if mice only experience light cycles of the equator, yet at temperatures of summer in the high temperate zones?
Reductionist experiments to measure the impact of abiotic environmental factors on immune function in mammals therefore inform both basic science and the prospects of translational impact. More of these studies are needed. And for all studies of lab mice, intentionality in experimental design—not just standardizing the abiotic environment, but also tailoring it to maximize relevance to real-world mammals—seems valuable.
Bringing the wild indoors: biotic factors, especially the littlest ones
Another reductionist approach to dissecting environmental drivers of immune phenotype entails experimental manipulation of biotic modifiers of the immune system. Here control groups of mice experience the usual conditions of research animal husbandry, while experimental groups diverge from those norms, but only in biotic environmental conditions.
For example, such experiments recently revealed impacts of the provision of exercise wheels (considering exercise here as an endogenous biotic factor) on the development of hematopoietic stem and progenitor cells (HSPCs)39 and type 2 immune responses17 in mice. Laboratory mice run avidly when given the opportunity40,41, putting even ultramarathoners to shame, when considering distance covered in relation to leg length! Such exercise arguably mimics wild foraging, fleeing and migratory behaviors, although the amount of exercise and the duress under which it is taken are likely very different in the lab and field. Might baseline and inducible immune activity in lab mice better model human immune activity if mice had regular access to exercise17? Similarly, given the well-understood impacts of diet on immune function (for example, see ref. 42), might a varied or seasonal diet for lab-reared mice make them more faithfully model human immune systems? Reductionist experiments to investigate how such aspects of the biotic environment alter the composition and responsiveness of the immune system remain crucial to informing whether, when and/or how we must naturalize lab mice.
However, as understanding of the potent effects of the microbiota on mammalian phenotypes has grown43, and with evidence that microbiota associated with different mouse breeders can chart divergent courses for otherwise-controlled experiments (for example, see ref. 44), many new insights into the immunological impact of the (micro)biotic environment are emerging. Specific-pathogen-free (SPF) conditions (for example, autoclaved cages and irradiated chow) remain the norm in animal husbandry, yet exposure to microbes appears to have tremendous potential to naturalize mouse models for immunology. Important recent work on ‘dirty mice’ has taken a number of creative directions (comprehensively and critically reviewed in ref. 45).
For example, co-housing lab mice with pet store mice46, providing lab mice with a history of infections47, giving lab mice fecal transplants from wild mice48 or even giving lab mice wild surrogate mothers49 can at least partly naturalize their immune systems. Mature CD8+ T cell fractions in the blood of lab-bred mice were especially naturalized by such antigenic experiences, and, accordingly, the mice became more resistant to microbial challenges46,47,48. In the case of the fascinating ‘wildling’ approach, exposure to the commensals and pathogens of a wild surrogate mother conferred persistent shifts to immune cell distributions and improved the match between mouse and human responses to anti-tumor necrosis factor (TNF) and CD28 superagonist treatments49. Specifically, wildlings were as impervious to treatment with anti-TNF monoclonal antibodies or TNF receptor–Fc fusion proteins during septic shock as people (for example, see ref. 3); they were also as susceptible to violent cytokine storms and failed regulatory T (Treg) cell expansion as human volunteers in an infamous clinical trial of a CD28 superagonist4. Lab mice in each case had failed to mimic the phenotype of the people, whereas the wildlings succeeded49.
These manipulations of antigenic experience suggest new approaches to experiments in immunology. The manipulations differ in their logistical difficulty as well as in their effects and effect sizes. Future work to unravel the route, dose and identity of microbes required to make murine and human immune systems best match (for example, for a given pathway of interest) is urgently needed45.
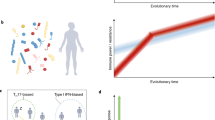
Yet what if a very complex cocktail of microbes and other antigens is required to induce the full array of interacting immune cells and functions of a wild animal? What if the effect size for antigenic experience on immune function is moderated by thermal conditions? Given that microbial colonizers often require dietary factors to exert their effect on the immune system (for example, to induce a circuit between tuft cells and innate lymphoid type 2 cells (ILC2s)50), what if a complex and varied diet expands the influence of environmentally acquired microbes? Finally, what if a broad suite of exposures is essential to cultivate the full array of helper T subsets (TH1, TH2, TH17, Treg and more) with which a wild animal is equipped? A complex world of concomitant abiotic and biotic environmental exposures may be essential for fully ‘natural’ immune responses (Fig. 1). Complementary, realistically complex approaches therefore also have a role to play in naturalization of mouse models.
Conceptually, we might think of the accrued environmental experience required to naturalize an inbred lab mouse as a game of Snakes and Ladders (also known as Chutes and Ladders). Every environmental experience that approximates that of wild animals (for example, antigenic experience or a seasonally varying photoperiod) functions like a ladder, taking the mouse closer to the environmentally naturalized immune state at the top right. Every artificial experience functions like a snake, taking the mouse back to its lab origins; it would be interesting to learn how long the effects of a natural environment might last upon return to controlled conditions. Examples of snakes and ladders here are of arbitrary placement and length and are for purposes of illustration only. Antigenic experience could include exposure to any infectious agents (from viruses to helminths) and symbionts, as well as allergens, and so son. A formal tabulation of abiotic and biotic factors affecting the immune system, and how those factors differ for wild and rewilded mice as compared to lab mice, is provided in ref. 35.
Bringing lab mice outdoors: greater realism, at the risk of greater complexity
Wild animals inhabit environments that clearly differ from lab conditions on multiple axes at once. Wild temperatures are more extreme and more variable than lab temperatures, wild microbes and parasite exposures are unpredictable in timing and dose and are hugely diverse as compared to lab exposures, and wild animals must forage, find shelter and otherwise look after themselves. What are the immunological consequences for lab mice that experience multivariate wild environments? A number of studies have taken lab mice into more natural environments, and even outdoors, to address this question.
For example, several studies in behavioral genetics have revealed insights into naturalized physiological systems of wild-derived, lab-bred mice maintained at high densities in barns, in ‘organismal performance assays’ (for example, see refs. 51,52). Such environments provide complex natural social conditions, including intense competitive interactions that reveal the profound metabolic impacts of vying for resources and mates51. How such competition relates to the stresses of life in a cage (especially when dominant individuals pick on subordinates) and whether it naturalizes immune function is an important area for further research. With the recent discovery that IL-6 acts as both inflammatory agent and stress hormone53, it seems likely that natural social stresses may directly modify immune phenotype.
Another vivid example is provided by Marilyn Scott’s work on inbred lab mice released into natural helminth transmission arenas. Scott found that the well-documented susceptibility of C57BL/6 mice to the helminth Heligmosomoides polygyrus disappeared when the mice were maintained in large peat-filled indoor arenas seeded with nematode larvae54. Extensive further work revealed that the peat substrate, low doses of larvae and ingesting larvae by eating (versus via gavage) had not caused the loss of susceptibility; instead, the slow natural rate of exposure allowed susceptible mice to become resistant55. Scott’s measurements focused on parasitology rather than immunology, but a recent paper comparing TH1-inducing large-dose ‘bolus’ infections to TH2-inducing repetitive small-dose ‘trickle’ infections with the helminth Trichuris muris56 suggests a role for differential immune response induction in explaining divergence between lab-dwellers and arena-dwellers. Doses, routes and rates of exposure that mimic realistic exposures of wild hosts may dramatically alter the induced immune response in lab mice (for example, low-dose intradermal infection is essential for the induction of CD8+ T cells by Leishmania major infection57). A natural epidemiological setting may thereby qualitatively change immune responses. Might rates of microbial exposure in the wild likewise make mice naturalized outdoors an important model to compare with mice given intense infection regimens (for example, see ref. 47) or a bolus of fecal transplant material (for example, see ref. 48)?
Our own work in this area entails ‘rewilding’ inbred mice, by maintaining them outdoors for several months, in a predator-free enclosure system (Fig. 2) originally built for studies of circadian rhythms58. This approach naturalizes the antigenic experience of the mice by allowing them to acquire microbes from the environment itself (for example, those present in soil, on plants and in rainwater), rather than from conspecifics (whether directly or via fecal transplant, as in the studies outlined in the previous section). Rewilded mice also experience otherwise-natural conditions such as opportunities to exercise (for example, digging burrows59 and moving about the pens at night35), varied photoperiod and circadian rhythms in temperature. We do, however, protect them from predation with tall enclosure walls, electric fencing, suspended aluminum plates, vegetation and small barns, and we provide them with feeding and watering stations. We developed live-trapping procedures to sample the mice at regular intervals and at experimental endpoints. In these replicate populations, we can control variables such as host genotype, age and exposure to conspecifics, to discover how exposure to the environment generates divergent immune phenotypes.
We find that, in comparison to littermates maintained in conventional SPF conditions, rewilded mice rapidly acquire diverse gut microbes60 and become more susceptible to gastrointestinal nematodes59 while maintaining body weight and overall condition35. These effects of rewilding are associated with profound changes to immune cell distributions59,61. For example, the proportion of mature, antigen-experienced T cells (CD62LloCD44hi CD8+ T cells) in rewilded mice parallels what is observed via the other microbial naturalization approaches outlined above (for example, CD44hi fractions in the ~75% of lab mice that survived co-housing with heavily infected pet store mice46). Comprehensive testing showed that rewilded mice had not been exposed to pathogens59,61, suggesting that their accrued antigenic experiences were instead driven by exposure to environmental microbes, in the context of other aspects of environmental realism, both abiotic and biotic (tabulated in ref. 35).
A chief disadvantage of rewilding is complexity that may imperil the reductionist understanding of immune responses that many immunologists seek, yet rewilding also brings several advantages. The first advantage is that control of genotype in a multivariate environment allows us to quantify the relative and interacting contributions of genes and environment to immune phenotype. In parallel, we can statistically dissect the contributions of genotype and environmental exposure to the acquisition of microbiota (as in ref. 62, but for mice kept outdoors). We can thereby assess whether pathways of large effect size in the lab are robust to the field. Although we have thus far only compared closely related individuals (that is, C57BL/6 mice and congenic knockouts at various immunogenetic loci), we find that environment swamps genetics, such that gene knockouts (including Stat6–/– and Nod2–/–) no longer have profound phenotypic effects (for example, see refs. 59,61); relatedly, it would be fascinating to discover the extent to which natural environments buffer the effects of human loss-of-function mutations63. More widely divergent murine genotypes (for example, in founder strains of the Collaborative Cross) are a priority for future study. In comparison to studies of fully wild animals (for example, mice with genetic and environmental contributors to immune variation8,9,64), the control of genetics by using inbred mice strengthens the ability to study how a multivariate environment moderates immune phenotype.
A second advantage of rewilding is that unexpected environmental factors can emerge as crucial drivers of immune phenotype. For example, although bacterial components are major contributors to gut microbiota diversity outdoors59,60, we also discovered that fungi are the most important microbial drivers of granulocyte expansion in rewilded mice65. Such discoveries in turn suggest new hypotheses that can be tested in subsequent reductionist experiments. Furthermore, the systemic effects of rewilding invite investigation of organs beyond the immune system that may nonetheless impact immune function: for example, neuronal growth in the hippocampus associated with learning behaviors and astrocyte activity outdoors66.
A third major advantage of rewilding is that it enables explicit study of immunological divergence among genetically related individuals that differ incrementally in environmental experiences. Crucially, mice outdoors have behavioral freedom that causes the microbial and other experiences of genetically matched individuals to diverge; immune phenotypes thus also diverge (analogously to what happens to human twins21). Time outdoors therefore expands variation among individuals, and this accrued environmental experience may leave idiosyncratic impacts on immune cells, as it does on neurons24. Still, rewilding generates repeatable and scientifically robust immunological findings. Indeed, across years, we find that rewilding repeatably expands mature T cells59,61 and granulocytes (ref. 65 and Y. H. Chen, F. Yeung, A. E. Downie, J. D. Lin, C. McCauley et al., manuscript in preparation).
It is important to note that, although t tests and other simple statistical approaches have their place in analysis of conventional experiments in immunology67, they are insufficient for analysis of complex drivers of immune phenotype in a realistic environment. Instead, rewilding and organismal performance arenas require more robust statistical tools to dissect complexity. For example, generalized linear mixed statistical models lend insight by quantifying the immunological variance independently or interactively associated with genotype and multiple environmental variables as well as sex, age and individual identity. Such statistical approaches are routinely used in wild immunology (for example, to reveal differential survival among Soay sheep exhibiting autoantibodies68 or immunosenescence69). When such statistics are combined with experimental designs that control for genotype, and even for aspects of environment, the power of the approach to dissect causes of immunophenotypic divergence is enhanced. For example, in our rewilding work, we can match the temperature, humidity and light–dark cycles in the lab to what mice are experiencing in the field, to control for abiotic factors that are not of central interest to our work. We can therefore exclude thermogenesis and account for initial body mass (using generalized linear mixed models) as causes of the altered T cell populations in the lamina propria of mice outdoors59.
Taking lab mice outdoors will never replace reductionist, highly controlled experiments, but holistic approaches still bring their own advantages. Given the potential for reductionist follow-up experiments after outdoor experiments, one need not choose between reductionism and holism. Besides, there are strong arguments for providing ‘a good life’ for lab rodents70 and for using studies on naturalized lab mice as an immunological bridge of understanding between fully caged and fully wild animals12,13,45.
Improving translational potential
Naturalizing lab-reared mice, whether by bringing elements of the natural world to them or by taking them outside, is expected to enable them to more closely resemble the structure (for example, in terms of relative abundances of different cell types) and therefore better model the function of human immune systems. As outlined above, for instance, we already know that providing wild surrogate mothers for lab mice renders the mice better models of inflammatory diseases and their treatments49. If this proves true more broadly, then naturalizing mouse models should make the immunology that they reveal translate better into improving human health45.
An emerging example centers on neutrophils, a classic ‘double-edged sword’ cell type, with powerful roles in killing microbes (for example, see refs. 71,72) and causing inflammatory damage (for example, see ref. 73). In the delicate tissue of the lung, neutrophils also promote tissue repair following injury, such that they have been targeted in clinical interventions—but to no avail thus far74, perhaps owing to poor modeling of the interactions between neutrophils and endothelial cells in conventional lab mice75. Indeed, neutropenia is a stark difference between conventional lab mice and the human immune system5,6,7. We were therefore struck by the dramatic increase in myeloid cells (especially neutrophils) in the blood and lymph nodes of rewilded mice that could be recreated under controlled lab conditions by inoculation with a wild fungus consortium, albeit with a smaller effect size than for rewilding per se65. It is exciting to speculate that the translational potential of mouse models of neutrophil-mediated immunity may be enhanced by logistically tractable fungal exposures back in conventional vivaria.
Naturalized mice suggest many other possibilities for improved translation, and it is sensible to encourage researchers to pursue the logistically easiest method that sufficiently naturalizes the aspect of immunity of interest45. The logistical challenges of taking lab mice outdoors59 or to another complex environment such as a barn51 may become essential when multiple physiological systems (from brain to liver) or multiple environmental axes (from housing temperature to microbial exposures) are of known importance to the immune phenotype of interest.
Mimicking natural variation in environmental experience could eventually become a routine step in the translational pipeline, as drugs or vaccines would need to pass muster outdoors (that is, be robust to environmental ‘noise’) in mice before proceeding to experiments in nonhuman primates and onward to clinical trials. A naturalized mouse step in the pipeline might save primate lives as well as time and money. For example, it is possible that immune pathways revealed by reductionist experiments will not predict phenotypes in a natural context. Drugs targeting those pathways may thus not prove effective in wild animals or people, even if all controlled lab experiments suggest that they ought to. It is important to note that a wild environment may either swamp or accentuate signals. If swamped, treatments might not work anymore. If accentuated, treatments might end up being dangerous. Field testing is routine in other industries (for example, in engineering) and is now advocated in toxicology (for example, see ref. 52); why not in immunology? Again, a literal field test may not be needed if a more tractable approach (for example, fecal transplants from wild mice) sufficiently naturalizes the target pathway45.
Further questions are raised by this line of thinking. For example, for what translational applications is it sufficient to nudge lab mice in the right direction toward wildness (for example, using selected environmental exposures to naturalize just one cell type or one anatomical location)? For what applications must lab mice more comprehensively mimic the phenotype of wild animals? Should naturalization be sought in species beyond mice (for example, colonies of nonhuman primates)? What can be translated from domesticated animals and even from fully wild ones, especially as we increasingly appreciate that our health is bound up with that of countless other animal species and now that comparative genomics tools open new vistas for discovery13? Finally, human populations live in a wide variety of environments12—for example, across a spectrum of exposures to worms and germs76—so might different types or extents of naturalization be needed to understand human immunology across that range? No matter the insights gleaned from other species, naturalized lab mice seem a promising part of the bridge from reductionist experiments in cellular and molecular immunology to the complex realities of human physiology.
Expanding evolutionary insights
Maizels and Nussey12 argued that research on a wide range of species across a spectrum of environments (from the lab bubble to the jungle) will be essential to understanding evolutionary causes of immunological variation. Flies13 proposed a research agenda that cycles iteratively from phylogenetic comparative analysis across vertebrates through pathway discovery and testing in dirty mice to field observations of wild animals and people, to broaden and deepen understanding of vertebrate immunology (akin to the productive iterations between phenomenological and mechanistic research in vaccinology that teach new immunology77). I second these calls. Much can be learned from comparative immunology across both environmental and taxonomic distances.
Questions arise for evolutionary biology as we contemplate environmental naturalization of lab mice. Two approaches immediately stand out. First, naturalized lab mice might elucidate selection pressures that have driven the evolution of mammalian immune defenses. For example, if certain branches of the immune system evolved to defend against opportunistic environmental microbes (for example, the inflammasome78,79), might exposing mice of divergent (including outbred) strains to a natural microbial environment facilitate the discovery of such mechanisms? Might environmental naturalization of lab mice also allow tests of the resilience of adult mammals raised in the lab bubble—for example, enabling experimental tests of phenotypic plasticity?
Second, wild immunology can suggest new hypotheses80 that might be usefully dissected in naturalized lab mice. For example, research in Cape buffalo has shown distinct immunological sequelae of natural versus drug-induced clearance of nematodes, with only the naturally resistant animals exhibiting potent type 2 immune effectors in lung tissue and therefore catastrophic susceptibility to tuberculosis81. This observation suggests distinct effects of genetic resistance and current helminth infection on the potency and location of cytokine cross-regulation during co-infection81. Mice have not yet been used to test this idea. Does TH1–TH2 cytokine cross-talk operate idiosyncratically in the buffalo or might a natural environment draw out variation among murine genotypes to make differential and tissue-specific immune feedbacks observable? No matter the answer, new insights into the evolution of cytokine signaling systems in mammals would emerge.
Conclusions
Multivariate environmental features, abiotic and biotic, likely shape the cellular composition and function of the immune system in lab mice—just as environment explains the bulk of human immune phenotype21,22,23. The experimental approaches to naturalize lab mice reviewed here offer new ways of honing understanding of mammalian immune function and applying that understanding to the development of vaccines and treatments. These approaches will never (and should never) totally replace experiments on controlled lab mouse genotypes in conventional, controlled environments. But neither should the current wave of interest in dirty45 and other naturalized mice be forced underground by fears that complex environments hamper mechanistic discovery. Arguably, research using conventional mouse husbandry prioritizes precision, which is of course valuable for discovery of immune mechanisms. But if environmental effects swamp or alter immune pathways, mechanisms understood only in the lab would not accurately reflect how immune systems really work. To avoid sacrificing accuracy in the name of precision, immunology research would be wise to keep going where the wild things are.
References
Viney, M. The gut microbiota of wild rodents: challenges and opportunities. Lab Anim. 53, 252–258 (2019).
Littman, D. R. Releasing the brakes on cancer immunotherapy. Cell 162, 1186–1190 (2015).
Fisher, C. J. Jr. et al. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. N. Engl. J. Med. 334, 1697–1702 (1996).
Suntharalingam, G. et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355, 1018–1028 (2006).
Mestas, J. & Hughes, C. C. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Masopust, D., Sivula, C. P. & Jameson, S. C. Of mice, dirty mice, and men: using mice to understand human immunology. J. Immunol. 199, 383–388 (2017).
Tao, L. & Reese, T. A. Making mouse models that reflect human immune responses. Trends Immunol. 38, 181–193 (2017).
Abolins, S. et al. The comparative immunology of wild and laboratory mice, Mus musculus domesticus. Nat. Commun. 8, 14811 (2017).
Abolins, S. et al. The ecology of immune state in a wild mammal, Mus musculus domesticus. PLoS Biol. 16, e2003538 (2018).
Mouse Genome Sequencing Consortium.Initial sequencing and comparative analysis of the mouse genome. Nature 420, 520–562 (2002).
Wade, C. M. et al. The mosaic structure of variation in the laboratory mouse genome. Nature 420, 574–578 (2002).
Maizels, R. M. & Nussey, D. H. Into the wild: digging at immunology’s evolutionary roots. Nat. Immunol. 14, 879–883 (2013).
Flies, A. S. & Wild Comparative Immunology Consortium. Rewilding immunology. Science 369, 37–38 (2020).
Davenport, M. P., Smith, N. L. & Rudd, B. D. Building a T cell compartment: how immune cell development shapes function. Nat. Rev. Immunol. 20, 499–506 (2020).
Puelma Touzel, M., Walczak, A. M. & Mora, T. Inferring the immune response from repertoire sequencing. PLoS Comput. Biol. 16, e1007873 (2020).
Onishi, K. G. et al. Circadian and circannual timescales interact to generate seasonal changes in immune function. Brain Behav. Immun. 83, 33–43 (2020).
Knudsen, N. H. et al. Interleukin-13 drives metabolic conditioning of muscle to endurance exercise. Science 368, eaat3987 (2020).
Procaccini, C., Pucino, V., De Rosa, V., Marone, G. & Matarese, G. Neuro-endocrine networks controlling immune system in health and disease. Front. Immunol. 5, 143 (2014).
Zhang, X. et al. Brain control of humoral immune responses amenable to behavioural modulation. Nature 581, 204–208 (2020).
Lopes, P. C., Carlitz, E. H. D., Kindel, M. & Konig, B. Immune–endocrine links to gregariousness in wild house mice. Front. Behav. Neurosci. 14, 10 (2020).
Brodin, P. et al. Variation in the human immune system is largely driven by non-heritable influences. Cell 160, 37–47 (2015).
Brodin, P. & Davis, M. M. Human immune system variation. Nat. Rev. Immunol. 17, 21–29 (2017).
Kaczorowski, K. J. et al. Continuous immunotypes describe human immune variation and predict diverse responses. Proc. Natl Acad. Sci. USA 114, E6097–E6106 (2017).
Freund, J. et al. Emergence of individuality in genetically identical mice. Science 340, 756–759 (2013).
Dall, S. R., Bell, A. M., Bolnick, D. I. & Ratnieks, F. L. An evolutionary ecology of individual differences. Ecol. Lett. 15, 1189–1198 (2012).
Speakman, J. R. & Keijer, J. Not so hot: optimal housing temperatures for mice to mimic the thermal environment of humans. Mol. Metab. 2, 5–9 (2012).
Hogberg, H. et al. Temperature dependence of O2 consumption; opposite effects of leptin and etomoxir on respiratory quotient in mice. Obesity 14, 673–682 (2006).
Fischer, A. W., Cannon, B. & Nedergaard, J. Optimal housing temperatures for mice to mimic the thermal environment of humans: an experimental study. Mol. Metab. 7, 161–170 (2018).
Karp, C. L. Unstressing intemperate models: how cold stress undermines mouse modeling. J. Exp. Med. 209, 1069–1074 (2012).
Ganeshan, K. & Chawla, A. Warming the mouse to model human diseases. Nat. Rev. Endocrinol. 13, 458–465 (2017).
Keijer, J., Li, M. & Speakman, J. R. What is the best housing temperature to translate mouse experiments to humans? Mol. Metab. 25, 168–176 (2019).
Blomqvist, A. & Engblom, D. Neural mechanisms of inflammation-induced fever. Neuroscientist 24, 381–399 (2018).
García, M. D. C., Pazos, P., Lima, L. & Diéguez, C. Regulation of energy expenditure and brown/beige thermogenic activity by interleukins: new roles for old actors. Int. J. Mol. Sci. 19, 2569 (2018).
Geltink, R. I. K., Kyle, R. L. & Pearce, E. L. Unraveling the complex interplay between T cell metabolism and function. Annu. Rev. Immunol. 36, 461–488 (2018).
Budischak, S. A. et al. Feeding immunity: physiological and behavioral responses to infection and resource limitation. Front. Immunol. 8, 1914 (2018).
Druzd, D. et al. Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses. Immunity 46, 120–132 (2017).
Hopwood, T. W. et al. The circadian regulator BMAL1 programmes responses to parasitic worm infection via a dendritic cell clock. Sci. Rep. 8, 3782 (2018).
Nobis, C. C. et al. The circadian clock of CD8 T cells modulates their early response to vaccination and the rhythmicity of related signaling pathways. Proc. Natl Acad. Sci. USA 116, 20077–20086 (2019).
Frodermann, V. et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat. Med. 25, 1761–1771 (2019).
Meijer, J. H. & Robbers, Y. Wheel running in the wild. Proc. Biol. Sci. 281, 20140210 (2014).
Goh, J. & Ladiges, W. Voluntary wheel running in mice. Curr. Protoc. Mouse Biol. 5, 283–290 (2015).
Tan, J. K. et al. Protein-coupled receptors—facilitators of diet-related immune regulation. Annu. Rev. Immunol. 35, 371–402 (2017).
McFall-Ngai, M. et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl Acad. Sci. USA 110, 3229–3236 (2013).
Villarino, N. F. et al. Composition of the gut microbiota modulates the severity of malaria. Proc. Natl Acad. Sci. USA 113, 2235–2240 (2016).
Hamilton, S. E. et al. New insights into the immune system using dirty mice. J. Immunol. 205, 3–11 (2020).
Beura, L. K. et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532, 512–516 (2016).
Reese, T. A. et al. Sequential infection with common pathogens promotes human-like immune gene expression and altered vaccine response. Cell Host Microbe 19, 713–719 (2016).
Rosshart, S. P. et al. Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell 171, 1015–1028 (2017).
Rosshart, S. P. et al. Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science 365, eaaw4361 (2019).
Schneider, C. et al. A metabolite-triggered tuft cell–ILC2 circuit drives small intestinal remodeling. Cell 174, 271–284 (2018).
Ruff, J. S. et al. Human-relevant levels of added sugar consumption increase female mortality and lower male fitness in mice. Nat. Commun. 4, 2245 (2013).
Gaukler, S. M. et al. Quantification of cerivastatin toxicity supports organismal performance assays as an effective tool during pharmaceutical safety assessment. Evol. Appl. 9, 685–696 (2016).
Qing, H. et al. Origin and function of stress-induced IL-6 in murine models. Cell 182, 372–387 (2020).
Scott, M. E. Heligmosomoides polygyrus (Nematoda): susceptible and resistant strains of mice are indistinguishable following natural infection. Parasitology 103, 429–438 (1991).
Scott, M. E. High transmission rates restore expression of genetically determined susceptibility of mice to nematode infections. Parasitology 132, 669–679 (2006).
Glover, M., Colombo, S. A. P., Thornton, D. J. & Grencis, R. K. Trickle infection and immunity to Trichuris muris. PLoS Pathog. 15, e1007926 (2019).
Belkaid, Y. et al. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J. Immunol. 168, 3992–4000 (2002).
Spoelstra, K., Wikelski, M., Daan, S., Loudon, A. S. & Hau, M. Natural selection against a circadian clock gene mutation in mice. Proc. Natl Acad. Sci. USA 113, 686–691 (2016).
Leung, J. M. et al. Rapid environmental effects on gut nematode susceptibility in rewilded mice. PLoS Biol. 16, e2004108 (2018).
Bar, J. et al. Strong effects of lab-to-field environmental transitions on the bacterial intestinal microbiota of Mus musculus are modulated by Trichuris muris infection. FEMS Microbiol. Ecol. 96, fiaa167 (2020).
Lin, J. D. et al. Rewilding Nod2 and Atg16l1 mutant mice uncovers genetic and environmental contributions to microbial responses and immune cell composition. Cell Host Microbe 27, 830–840 (2020).
Benson, A. K. et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc. Natl Acad. Sci. USA 107, 18933–18938 (2010).
MacArthur, D. G. et al. A systematic survey of loss-of-function variants in human protein-coding genes. Science 335, 823–828 (2012).
Taylor, C. H. et al. Immune state is associated with natural dietary variation in wild mice Mus musculus domesticus. Funct. Ecol. 33, 1425–1435 (2019).
Yeung, F. et al. Altered immunity of laboratory mice in the natural environment is associated with fungal colonization. Cell Host Microbe 27, 809–822 (2020).
Cope, E. C. et al. The effects of living in an outdoor enclosure on hippocampal plasticity and anxiety-like behavior in response to nematode infection. Hippocampus 29, 366–377 (2019).
Lamb, T. J., Graham, A. L. & Petrie, A. t-Testing the immune system. Immunity 28, 288–292 (2008).
Graham, A. L. et al. Fitness correlates of heritable variation in antibody responsiveness in a wild mammal. Science 330, 662–665 (2010).
Froy, H. et al. Senescence in immunity against helminth parasites predicts adult mortality in a wild mammal. Science 365, 1296–1298 (2019).
Makowska, I. J. & Weary, D. M. A good life for laboratory rodents? ILAR J. https://doi.org/10.1093/ilar/ilaa001 (2020).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Branzk, N. et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 15, 1017–1025 (2014).
Knackstedt, S. L. et al. Neutrophil extracellular traps drive inflammatory pathogenesis in malaria. Sci. Immunol. 4, eaaw0336 (2019).
Blazquez-Prieto, J., Lopez-Alonso, I., Huidobro, C. & Albaiceta, G. M. The emerging role of neutrophils in repair after acute lung injury. Am. J. Respir. Cell Mol. Biol. 59, 289–294 (2018).
Soroush, F. et al. Neutrophil–endothelial interactions of murine cells is not a good predictor of their interactions in human cells. FASEB J. 34, 2691–2702 (2020).
Ramanan, D. et al. Helminth infection promotes colonization resistance via type 2 immunity. Science 352, 608–612 (2016).
Pulendran, B., Li, S. & Nakaya, H. I. Systems vaccinology. Immunity 33, 516–529 (2010).
Maltez, V. I. et al. Inflammasomes coordinate pyroptosis and natural killer cell cytotoxicity to clear infection by a ubiquitous environmental bacterium. Immunity 43, 987–997 (2015).
Maltez, V. I. & Miao, E. A. Reassessing the evolutionary importance of inflammasomes. J. Immunol. 196, 956–962 (2016).
Pedersen, A. B. & Babayan, S. A. Wild immunology. Mol. Ecol. 20, 872–880 (2011).
Ezenwa, V. O. et al. Natural resistance to worms exacerbates bovine tuberculosis severity independently of worm coinfection. Proc. Natl Acad. Sci. USA 118, e2015080118 (2020).
Acknowledgements
Hearty thanks to K. Cadwell, W. Craigens, P. Loke and L. Wait for helpful comments on drafts.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peer review information Zoltan Fehervari was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Rights and permissions
About this article
Cite this article
Graham, A.L. Naturalizing mouse models for immunology. Nat Immunol 22, 111–117 (2021). https://doi.org/10.1038/s41590-020-00857-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-020-00857-2
This article is cited by
-
Female behavior drives the formation of distinct social structures in C57BL/6J versus wild-derived outbred mice in field enclosures
BMC Biology (2024)
-
Development of HPV16 mouse and dog models for more accurate prediction of human vaccine efficacy
Laboratory Animal Research (2023)
-
Microbial experience through housing in a farmyard-type environment alters intestinal barrier properties in mouse colons
Scientific Reports (2023)
-
Commentary: Infectious disease — the ecological theater and the evolutionary play
Evolutionary Ecology (2023)
-
Cancer nanomedicine
Nature Reviews Cancer (2022)