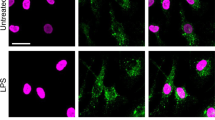
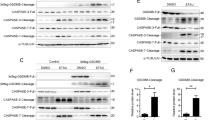
Abstract
Inflammatory caspase sensing of cytosolic lipopolysaccharide (LPS) triggers pyroptosis and the concurrent release of damage-associated molecular patterns (DAMPs). Collectively, DAMPs are key determinants that shape the aftermath of inflammatory cell death. However, the identity and function of the individual DAMPs released are poorly defined. Our proteomics study revealed that cytosolic LPS sensing triggered the release of galectin-1, a β-galactoside-binding lectin. Galectin-1 release is a common feature of inflammatory cell death, including necroptosis. In vivo studies using galectin-1-deficient mice, recombinant galectin-1 and galectin-1-neutralizing antibody showed that galectin-1 promotes inflammation and plays a detrimental role in LPS-induced lethality. Mechanistically, galectin-1 inhibition of CD45 (Ptprc) underlies its unfavorable role in endotoxin shock. Finally, we found increased galectin-1 in sera from human patients with sepsis. Overall, we uncovered galectin-1 as a bona fide DAMP released as a consequence of cytosolic LPS sensing, identifying a new outcome of inflammatory cell death.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The RNA-seq data have been deposited with the Gene Expression Omnibus (accession number GSE140892). Source data and uncropped immunoblot images are included in the paper as supplementary information. All other data supporting the findings of the paper are available from the corresponding author upon reasonable request.
References
Rathinam, V. A. K. & Fitzgerald, K. A. Inflammasome complexes: emerging mechanisms and effector functions. Cell 165, 792–800 (2016).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013).
Vanaja, S. K. et al. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell 165, 1106–1119 (2016).
Rathinam, V. A. K., Zhao, Y. & Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 20, 527–533 (2019).
Cheng, K. T. et al. Caspase-11-mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. J. Clin. Invest. 127, 4124–4135 (2017).
Kang, R. et al. Lipid peroxidation drives gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe 24, 97–108.e4 (2018).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
Song, F. et al. Sphingosine-1-phosphate receptor 2 signaling promotes caspase-11–dependent macrophage pyroptosis and worsens Escherichia coli sepsis outcome. Anesthesiology 129, 311–320 (2018).
Lamkanfi, M. et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 185, 4385–4392 (2010).
Wang, H. et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 (1999).
Keller, M., Rüegg, A., Werner, S. & Beer, H.-D. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818–831 (2008).
Sundblad, V., Morosi, L. G., Geffner, J. R. & Rabinovich, G. A. Galectin-1: a jack-of-all-trades in the resolution of acute and chronic inflammation. J. Immunol. 199, 3721–3730 (2017).
Camby, I., Le Mercier, M., Lefranc, F. & Kiss, R. Galectin-1: a small protein with major functions. Glycobiology 16, 137R–157R (2006).
Lee, H.-J. et al. Application of a peptide‐based PF2D platform for quantitative proteomics in disease biomarker discovery. Proteomics 8, 3371–3381 (2008).
Banerjee, I. et al. Gasdermin D restrains type I interferon response to cytosolic DNA by disrupting ionic homeostasis. Immunity 49, 413–426.e5 (2018).
Carta, S., Lavieri, R. & Rubartelli, A. Different members of the IL-1 family come out in different ways: DAMPs vs. cytokines? Front. Immunol. 4, 123 (2013).
Piccioli, P. & Rubartelli, A. The secretion of IL-1β and options for release. Semin. Immunol. 25, 425–429 (2013).
Evavold, C. L. et al. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48, 35–44.e6 (2018).
Chen, Y. et al. Gasdermin D drives the nonexosomal secretion of galectin-3, an insulin signal antagonist. J. Immunol. 203, 2712–2723 (2019).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016).
Frank, D. & Vince, J. E. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 26, 99–114 (2019).
Galluzzi, L., Kepp, O., Chan, F. K.-M. & Kroemer, G. Necroptosis: mechanisms and relevance to disease. Annu. Rev. Pathol. 12, 103–130 (2017).
Delano, M. J. & Ward, P. A. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J. Clin. Invest. 126, 23–31 (2016).
Zhang, W. & Coopersmith, C. M. Dying as a pathway to death in sepsis. Anesthesiology 129, 238–240 (2018).
Mandal, P. et al. Caspase-8 collaborates with caspase-11 to drive tissue damage and execution of endotoxic shock. Immunity 49, 42–55.e6 (2018).
Schenck, E. J. et al. Circulating cell death biomarker TRAIL is associated with increased organ dysfunction in sepsis. JCI Insight 4, e127143 (2019).
Gong, T., Liu, L., Jiang, W. & Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases.Nat. Rev. Immunol. 20, 95–112 (2020).
Rabinovich, G. A. & Toscano, M. A. Turning ‘sweet’ on immunity: galectin–glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 9, 338–352 (2009).
Earl, L. A., Bi, S. & Baum, L. G. N- and O-glycans modulate galectin-1 binding, CD45 signaling, and T cell death. J. Biol. Chem. 285, 2232–2244 (2010).
Walzel, H., Schulz, U., Neels, P. & Brock, J. Galectin-1, a natural ligand for the receptor-type protein tyrosine phosphatase CD45. Immunol. Lett. 67, 193–202 (1999).
Irie-Sasaki, J. et al. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature 409, 349–354 (2001).
Piercy, J., Petrova, S., Tchilian, E. Z. & Beverley, P. C. L. CD45 negatively regulates tumour necrosis factor and interleukin-6 production in dendritic cells. Immunology 118, 250–256 (2006).
Zanoni, I. et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 352, 1232–1236 (2016).
Wolf, A. J. et al. Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell 166, 624–636 (2016).
Lei, T. et al. Galectin-1 enhances TNFα-induced inflammatory responses in Sertoli cells through activation of MAPK signalling. Sci. Rep. 8, 3741 (2018).
Pérez, C. V. et al. Dual roles of endogenous and exogenous galectin-1 in the control of testicular immunopathology. Sci. Rep. 5, 12259 (2015).
Toegel, S. et al. Galectin-1 couples glycobiology to inflammation in osteoarthritis through the activation of an NF-κB-regulated gene network. J. Immunol. 196, 1910–1921 (2016).
Starossom, S. C. et al. Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity 37, 249–263 (2012).
Huang, X.-T. et al. Galectin-1 ameliorates lipopolysaccharide-induced acute lung injury via AMPK-Nrf2 pathway in mice. Free Radic. Biol. Med. 146, 222–233 (2020).
Ge, X. N. et al. Regulation of eosinophilia and allergic airway inflammation by the glycan-binding protein galectin-1. Proc. Natl Acad. Sci. USA 113, E4837–E4846 (2016).
Rodrigues, L. C. et al. Galectin-1 modulation of neutrophil reactive oxygen species production depends on the cell activation state. Mol. Immunol. 116, 80–89 (2019).
Croci, D. O. et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell 156, 744–758 (2014).
Di Lella, S. et al. When galectins recognize glycans: from biochemistry to physiology and back again. Biochemistry 50, 7842–7857 (2011).
Poirier, F. & Robertson, E. J. Normal development of mice carrying a null mutation in the gene encoding the L14 S-type lectin. Development 119, 1229–1236 (1993).
Martínez Allo, V. C. et al. Suppression of age-related salivary gland autoimmunity by glycosylation-dependent galectin-1-driven immune inhibitory circuits. Proc. Natl Acad. Sci. USA 117, 6630–6639 (2020).
Rathinam, V. A. K. et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by Gram-negative bacteria. Cell 150, 606–619 (2012).
Ménoret, A. et al. The oxazolidinone derivative locostatin induces cytokine appeasement. J. Immunol. 183, 7489–7496 (2009).
Fettis, M. M. & Hudalla, G. A. Engineering reactive oxygen species-resistant galectin-1 dimers with enhanced lectin activity. Bioconjug. Chem. 29, 2489–2496 (2018).
Pérez Sáez, J. M. et al. Characterization of a neutralizing anti-human galectin-1 monoclonal antibody with angioregulatory and immunomodulatory activities. Angiogenesis https://doi.org/10.1007/s10456-020-09749-3 (2020).
Bone, R. C. et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 101, 1644–1655 (1992).
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810 (2016).
Acknowledgements
We thank J. Conejo-Garcia for the Lgals1−/− mice and V. Dixit (Genentech) and K. Fitzgerald (University of Massachusetts Medical School) for the Casp11−/− and Gsdmd−/− mice, S. Yeung and K. Khanna for help with the multiplex analysis and G. Fong for the MS1 cell line (University of Connecticut Health). This work was supported by the National Institutes of Health (grant nos. AI119015 and AI135528 to V.A.R.); Agencia Nacional de Promoción Científica y Tecnológica, Fundación Sales and Fundación Bunge y Born to G.A.R.; the Federal Ministry of Education and Research, Germany (grant no. 01EO1502) and Deutsche Forschungsgemeinschaft (German Research Foundation) under Germany’s Excellence Strategy-EXC 2051, project ID 390713860 (to M.B.).
Author information
Authors and Affiliations
Contributions
V.A.R. and A.J.R. conceived the study. V.A.R., G.A.R. and A.J.R. designed the experiments and wrote the manuscript. A.J.R., S.O.V., A.M., S.P.M.H., P.K., J.M.P.S., C.W., K.C., S.K.V., J.R., G.A.R. and A.T.V. performed the experiments, analyzed the data or provided technical or conceptual help. S.D., S.D.D., M.B. and C.S. performed the human sample analysis. M.M.F., R.L., A.W. and G.A.H. provided the recombinant galectin-1 and GFP. B.Z. and C.L. performed the RNA-seq analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Immunology thanks Derek Abbott and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Zoltan Fehervari was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Galectin-1 is released as a consequence of inflammasome activation.
a, Experimental design for profiling caspase-11-mediated release of DAMPs by PF2D-based proteomics. WT and Casp11–/– BMDMs were infected with EHEC (MOI=50) for 16 h and supernatants were harvested and concentrated and processed on the Beckman Coulter ProteomeLab PF2D platform. b, Galectin-1 secretion by Pam3CSK4-primed WT and Lgals1–/– BMDMs stimulated with EHEC (MOI=50) or poly(dA:dT) transfection for 16 h or 10 µM nigericin for 1 h. c, Immunoblot of galectin-1 (14.5 kDa) in the supernatant and lysates of BMDMs stimulated as in (b). d, IL-1α secretion by Pam3CSK4-primed WT, Casp11–/–, and Casp1–/– BMDMs stimulated with 10 μM nigericin for 1 h. e, Immunoblot of caspase-11 and β-actin in lysates of MS1 endothelial cells transduced with LentiCRISPR V2 plasmid expressing control (GFP) sgRNA or caspase-11 sgRNA. f, Immunoblot of caspase-4 and β-actin in lysates of HeLa cells transduced with LentiCRISPR V2 plasmid expressing control (GFP) sgRNA or caspase-4 sgRNA. g, Immunoblot of GSDMD in the lysates of Pam3CSK4-primed WT BMDMs infected with EHEC or S. flexneri (MOI=50) for 16 h or 10 µM nigericin for 1 h in the presence or absence of 50 mM glycine. h, Galectin-1 release from liposomes packaged with galectin-1 and incubated with either 1 µg recombinant active caspase-11, 2 µg recombinant gasdermin D, both caspase-11 and gasdermin D, or 0.1% Triton X-100. Data are presented as mean ± SEM of one experiment representative of two (b,h; h is the replicate for the Fig. 2i). Combined data from two independent experiments (d) are shown as mean ± SEM. Immunoblots (c,e–g) are representative of two independent experiments.
Extended Data Fig. 2 Galectin-1 contributes to lethality during sepsis.
a, Plasma amounts of ALT and LDH in WT, Lgals1–/–, Casp11–/–, and Gsdmd–/– mice injected i.p. with 5 mg/kg LPS for 18 h. b, Survival of WT (n=9), Lgals1–/– (n=9), and Casp11–/– mice (n=4) injected i.p. with E. coli (5x108 CFU). c, Survival of Lgals1–/– mice injected i.p. with LPS (3 mg/kg) followed by i.p. injection of PBS (n=4), 100 µg of recombinant GFP (n=4), or 100 µg rGal-1 (n=4) 1 h later. d, Survival of Lgals1–/– mice injected i.p. with LPS (3 mg/kg) followed by i.p. injection of 100 µg of rGal-1 (n=6) or heat inactivated rGal-1 (HI rGal-1) (n=6) 1 h later. e, TNF and IL-6 secretion by WT BMDMs stimulated with 5 µM rGal-1 or 1 µg LPS for 16 h. f, Survival of WT and Casp11–/– mice injected i.p. with PBS (n=3) or 100 µg of rGal-1 (n=3). Combined data from two independent experiments are shown (b,d, and e). In (e) data are presented as mean ± SEM. In (a), each circle represents a mouse and the horizontal lines represent mean. ns, not significant (one-way ANOVA). *p < 0.05, one-way ANOVA (a); Mantel-Cox test (d).
Extended Data Fig. 3 Galectin-1 amplifies systemic inflammatory responses during LPS shock.
a,b, Cytokine and chemokine levels in the lung (a) and spleen (b) homogenates of WT and Lgals1–/– mice injected i.p. with 5 mg/kg LPS for 20 h. Combined data from two independent experiments are shown. Each circle represents a mouse and the horizontal lines represent mean. *p < 0.05; unpaired two-tailed t test (a–b); ns, not significant.
Extended Data Fig. 4 Galectin-1 amplifies systemic inflammatory responses during endotoxemia.
a, Indicated cytokine and chemokine levels in the plasma of WT (n=5), Lgals1–/– (n=5), Casp11–/– (n=5), and Gsdmd–/– (n=5) mice injected i.p. with 5 mg/kg LPS for 20 h. Each circle represents a mouse and the horizontal lines represent mean. *p < 0.05; one-way ANOVA followed by Sidak’s post-test.
Extended Data Fig. 5 Galectin-1 functions in a glycan-dependent manner during endotoxemia.
a, Survival of WT, Mgat5–/–, and C2gnt1–/– mice injected i.p. with 5 mg/kg LPS followed by i.p. injection of PBS or 100 µg of rGal-1 1 h later (WT LPS PBS n=6, WT LPS rGal-1 n=7, Mgat5–/– LPS PBS n=5, Mgat5–/– LPS rGal-1 n=5, C2gnt1–/– LPS PBS n=9 and C2gnt1–/– LPS rGal-1 n=11). b, Plasma amounts of ALT and LDH in WT (n=6), Mgat5–/– (n=5), and C2gnt1–/– (n=9) mice injected i.p. with 5 mg/kg LPS for 18 h. c, Galectin-1 levels in the plasma of WT (n=6), Mgat5–/– (n=5), and C2gnt1–/– (n=9) mice injected i.p. with 5 mg/kg LPS for 18 h. d, Immunoblot of CD45 that was immunoprecipitated with anti-CD45 antibody or IgG control antibody from the spleen homogenates of WT mice. Each circle represents a mouse and the horizontal lines represent mean (b,c). ns, not significant; one-way ANOVA followed by the Sidak’s post-test. Immunoblots (d) are representative of two independent experiments (d).
Supplementary information
Supplementary Information
Supplementary Table 1.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Uncropped immunoblot image.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Uncropped immunoblot image.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Uncropped immunoblot image.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 7
Uncropped immunoblot image.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Uncropped immunoblot image.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Uncropped immunoblot image.
Rights and permissions
About this article
Cite this article
Russo, A.J., Vasudevan, S.O., Méndez-Huergo, S.P. et al. Intracellular immune sensing promotes inflammation via gasdermin D–driven release of a lectin alarmin. Nat Immunol 22, 154–165 (2021). https://doi.org/10.1038/s41590-020-00844-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-020-00844-7
This article is cited by
-
The gasdermin family: emerging therapeutic targets in diseases
Signal Transduction and Targeted Therapy (2024)
-
Circulating tumor cells shielded with extracellular vesicle-derived CD45 evade T cell attack to enable metastasis
Signal Transduction and Targeted Therapy (2024)
-
Galectin-1-dependent ceRNA network in HRMECs revealed its association with retinal neovascularization
BMC Genomics (2023)
-
Oxidized mitochondrial DNA induces gasdermin D oligomerization in systemic lupus erythematosus
Nature Communications (2023)
-
Targeting galectin-driven regulatory circuits in cancer and fibrosis
Nature Reviews Drug Discovery (2023)