Abstract
The initial response to viral infection is anticipatory, with host antiviral restriction factors and pathogen sensors constantly surveying the cell to rapidly mount an antiviral response through the synthesis and downstream activity of interferons. After pathogen clearance, the host’s ability to resolve this antiviral response and return to homeostasis is critical. Here, we found that isoforms of the RNA-binding protein ZAP functioned as both a direct antiviral restriction factor and an interferon-resolution factor. The short isoform of ZAP bound to and mediated the degradation of several host interferon messenger RNAs, and thus acted as a negative feedback regulator of the interferon response. In contrast, the long isoform of ZAP had antiviral functions and did not regulate interferon. The two isoforms contained identical RNA-targeting domains, but differences in their intracellular localization modulated specificity for host versus viral RNA, which resulted in disparate effects on viral replication during the innate immune response.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Schoggins, J. W. et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472, 481–485 (2011).
Harris, R. S. & Dudley, J. P. APOBECs and virus restriction. Virology 479-480, 131–145 (2015).
Haller, O., Staeheli, P., Schwemmle, M. & Kochs, G. Mx GTPases: dynamin-like antiviral machines of innate immunity. Trends Microbiol. 23, 154–163 (2015).
Diamond, M. S. & Farzan, M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat. Rev. Immunol. 13, 46–57 (2013).
Jarret, A. et al. Hepatitis-C-virus-induced microRNAs dampen interferon-mediated antiviral signaling. Nat. Med. 22, 1475–1481 (2016).
McFarland, A. P. et al. The favorable IFNL3 genotype escapes mRNA decay mediated by AU-rich elements and hepatitis C virus-induced microRNAs. Nat. Immunol. 15, 72–79 (2014).
Savan, R. Post-transcriptional regulation of interferons and their signaling pathways. J. Interferon Cytokine Res. 34, 318–329 (2014).
Schwerk, J. & Savan, R. Translating the untranslated region. J. Immunol. 195, 2963–2971 (2015).
Bick, M. J. et al. Expression of the zinc-finger antiviral protein inhibits alphavirus replication. J. Virol. 77, 11555–11562 (2003).
Gao, G., Guo, X. & Goff, S. P. Inhibition of retroviral RNA production by ZAP, a CCCH-type zinc finger protein. Science 297, 1703–1706 (2002).
Muller, S. et al. Inhibition of filovirus replication by the zinc finger antiviral protein. J. Virol. 81, 2391–2400 (2007).
Zhu, Y. et al. Zinc-finger antiviral protein inhibits HIV-1 infection by selectively targeting multiply spliced viral mRNAs for degradation. Proc. Natl Acad. Sci. USA 108, 15834–15839 (2011).
Guo, X., Carroll, J. W., Macdonald, M. R., Goff, S. P. & Gao, G. The zinc finger antiviral protein directly binds to specific viral mRNAs through the CCCH zinc finger motifs. J. Virol. 78, 12781–12787 (2004).
Guo, X., Ma, J., Sun, J. & Gao, G. The zinc-finger antiviral protein recruits the RNA processing exosome to degrade the target mRNA. Proc. Natl Acad. Sci. USA 104, 151–156 (2007).
Kerns, J. A., Emerman, M. & Malik, H. S. Positive selection and increased antiviral activity associated with the PARP-containing isoform of human zinc-finger antiviral protein. PLoS Genet. 4, e21 (2008).
Vyas, S., Chesarone-Cataldo, M., Todorova, T., Huang, Y. H. & Chang, P. A systematic analysis of the PARP protein family identifies new functions critical for cell physiology. Nat. Commun. 4, 2240 (2013).
Hayakawa, S. et al. ZAPS is a potent stimulator of signaling mediated by the RNA helicase RIG-I during antiviral responses. Nat. Immunol. 12, 37–44 (2011).
Ryman, K. D. et al. Sindbis virus translation is inhibited by a PKR/RNase l-independent effector induced by alpha/beta interferon priming of dendritic cells. J. Virol. 79, 1487–1499 (2005).
Wang, N. et al. Viral induction of the zinc finger antiviral protein is IRF3-dependent but NF-κB-independent. J. Biol. Chem. 285, 6080–6090 (2010).
Takagaki, Y., Seipelt, R. L., Peterson, M. L. & Manley, J. L. The polyadenylation factor CstF-64 regulates alternative processing of IgM heavy chain pre-mRNA during B cell differentiation. Cell 87, 941–952 (1996).
Chuvpilo, S. et al. Alternative polyadenylation events contribute to the induction of NF-ATc in effector T cells. Immunity 10, 261–269 (1999).
Shell, S. A., Hesse, C., Morris, S. M. Jr. & Milcarek, C. Elevated levels of the 64-kDa cleavage stimulatory factor (CstF-64) in lipopolysaccharide-stimulated macrophages influence gene expression and induce alternative poly(A) site selection. J. Biol. Chem. 280, 39950–39961 (2005).
Lee, H. et al. Zinc-finger antiviral protein mediates retinoic acid inducible gene I-like receptor-independent antiviral response to murine leukemia virus. Proc. Natl Acad. Sci. USA 110, 12379–12384 (2013).
Charron, G., Li, M. M., MacDonald, M. R. & Hang, H. C. Prenylome profiling reveals S-farnesylation is crucial for membrane targeting and antiviral activity of ZAP long-isoform. Proc. Natl Acad. Sci. USA 110, 11085–11090 (2013).
Wang, M. & Casey, P. J. Protein prenylation: unique fats make their mark on biology. Nat. Rev. Mol. Cell Biol. 17, 110–122 (2016).
Gustafsson, M. G. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 198, 82–87 (2000).
Zhang, Y., Burke, C. W., Ryman, K. D. & Klimstra, W. B. Identification and characterization of interferon-induced proteins that inhibit alphavirus replication. J. Virol. 81, 11246–11255 (2007).
Kujala, P. et al. Biogenesis of the Semliki Forest virus RNA replication complex. J. Virol. 75, 3873–3884 (2001).
DiCiommo, D. P. & Bremner, R. Rapid, high level protein production using DNA-based Semliki Forest virus vectors. J. Biol. Chem. 273, 18060–18066 (1998).
Chiu, H. P. et al. Inhibition of Japanese encephalitis virus infection by the host zinc-finger antiviral protein. PLoS Pathog. 14, e1007166 (2018).
Zhu, Y. & Gao, G. ZAP-mediated mRNA degradation. RNA Biol. 5, 65–67 (2008).
Zhu, Y., Wang, X., Goff, S. P. & Gao, G. Translational repression precedes and is required for ZAP-mediated mRNA decay. EMBO J. 31, 4236–4246 (2012).
Rosengren, A. T., Nyman, T. A., Syyrakki, S., Matikainen, S. & Lahesmaa, R. Proteomic and transcriptomic characterization of interferon-α-induced human primary T helper cells. Proteomics 5, 371–379 (2005).
Huang, Z., Wang, X. & Gao, G. Analyses of SELEX-derived ZAP-binding RNA aptamers suggest that the binding specificity is determined by both structure and sequence of the RNA. Protein Cell 1, 752–759 (2010).
Takata, M. A. et al. CG dinucleotide suppression enables antiviral defence targeting non-self RNA. Nature 550, 124–127 (2017).
Chen, S. et al. Structure of N-terminal domain of ZAP indicates how a zinc-finger protein recognizes complex RNA. Nat. Struct. Mol. Biol. 19, 430–435 (2012).
Glasker, S., Toller, M. & Kummerer, B. M. The alternate triad motif of the poly(ADP-ribose) polymerase-like domain of the human zinc finger antiviral protein is essential for its antiviral activity. J. Gen. Virol. 95, 816–822 (2014).
Liu, C. H., Zhou, L., Chen, G. & Krug, R. M. Battle between influenza A virus and a newly identified antiviral activity of the PARP-containing ZAPL protein. Proc. Natl Acad. Sci. USA 112, 14048–14053 (2015).
Wang, X. L. et al. Sindbis virus can exploit a host antiviral protein to evade immune surveillance. J. Virol. 90, 10247–10258 (2016).
Saito, T., Owen, D. M., Jiang, F., Marcotrigiano, J. & Gale, M. Jr. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454, 523–527 (2008).
Urban, T. J. et al. IL28B genotype is associated with differential expression of intrahepatic interferon-stimulated genes in patients with chronic hepatitis C. Hepatology 52, 1888–1896 (2010).
Friedman, J. R., Webster, B. M., Mastronarde, D. N., Verhey, K. J. & Voeltz, G. K. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J. Cell Biol. 190, 363–375 (2010).
Rowland, A. A., Chitwood, P. J., Phillips, M. J. & Voeltz, G. K. ER contact sites define the position and timing of endosome fission. Cell 159, 1027–1041 (2014).
Olenych, S. G., Claxton, N. S., Ottenberg, G. K. & Davidson, M. W. The fluorescent protein color palette. Curr. Protoc. Cell Biol. 33, 21.5.25 (2006).
Vogt, D. A. & Ott, M. Membrane flotation assay. Bio Protoc. 5, e1435 (2015).
Boritz, E., Gerlach, J., Johnson, J. E. & Rose, J. K. Replication-competent rhabdoviruses with human immunodeficiency virus type 1 coats and green fluorescent protein: entry by a pH-independent pathway. J. Virol. 73, 6937–6945 (1999).
Acknowledgements
This project was funded by National Institutes of Health grants (nos. AI108765 and AI135437 to R.S.; no. AI119017 to M.D.D.; and nos. AI104002, AI118916 and AI127463 to M.G.); the Pew Biomedical Scholars program (no. 32011 to M.D.D.); a Research Fellowship from the German Research Foundation (no. SCHW 1881/1-1 to J.S.); T32 training grants (nos. AI106677 and GM007270 to F.W.S.); and T32 training grant (no. GM007240 to A.P.R.). This work was supported in part by the UW Proteomics Resource (UWPR95794). We thank P. von Haller and J. Eng (UW Proteomics Resource) for expert technical assistance with mass spectrometry. We are thankful for the support of W. P. Chang (UW Biology Imaging Facility) for help with super-resolution microscopy. We thank M. A. Davis (UW Immunology) for help with confocal laser scanning microscopy, D. B. Stetson and K. Burleigh (UW Immunology) for providing the IRF3 CRISPR construct, S. N. Sarkar (University of Pittsburgh) for providing STAT1 KO PH5CH8 cells and members of the Savan, Daugherty and Gale laboratories for helpful discussions.
Author information
Authors and Affiliations
Contributions
J.S., F.W.S., M.G., M.D.D. and R.S. designed the study. R.S. directed the study. J.S., F.W.S., A.P.R., K.R.T., L.D.H., S.O., A.F., A.M.K., J.A.R., L.S. and J.L.H. performed experiments and analyzed the data. J.S., M.D.D. and R.S. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Ioana Visan was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 ZAP-S interacts with the 3′UTR of IFN mRNAs.
(a) ZAP RNA-IP performed in Huh7 ZAP KO cells under UV crosslinking conditions after expression of FLAG-tagged ZAP-S, FLAG-tagged ZAP-L and FLAG-empty vector (EV) control, and stimulation with 0.2 µg/ml RIG-I ligand for 24 h. (b) RNA-IP of endogenous ZAP performed in Huh7 WT and IFNAR1 KO cells after stimulation with 0.5 µg/ml RIG-I ligand for 24 h. Representative experiments of (a) two and (b) three independently performed experiments with similar results are shown.
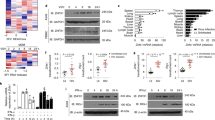
Supplementary Figure 2 ZAP-deficient cells have a higher and more prolonged IFN response.
(a) Western blot of Huh7 ZAP KO cell pools generated by CRISPR-Cas9 technology using two different guide RNAs. (b) ZAP mRNA expression in ZAP KO cell pools. (c) IFNB and IFNL3 mRNA expression in ZAP KO cell pools compared to WT cells and a single-cell sub-clone after stimulation with RIG-I ligand for 24 h. (a-c) Representative experiments with replicates (n = 3) of two independently performed experiments with similar results are shown. Bars show mean ± SD. (d) Sequencing chromatogram of the CRISPR target region in exon 1 of the human ZAP (ZC3HAV1) gene. Four bacterial clones were sequenced showing the same del C deletion. (e) Alignment of WT and CRISPR targeted ZAP polypeptide sequence (amino acid residue 1-200). The ΔC base pair deletion results in a premature stop codon at amino acid residue 121 (exon 2). (f) Polysome profiling of HPRT and IFNB mRNA after overexpression of ZAP-S (green), ZAP-L (blue), or empty vector (EV, black) control in Huh7 ZAP KO cells and stimulation of the cells with 0.25 µg/ml RIG-I ligand for 18 h. A representative of two independent experiments with similar results is shown. Data were analyzed using one-way ANOVA with Tukey’s post-test. *P < 0.01; **P < 0.001; ns, not significant.
Supplementary Figure 3 ZAP-S and ZAP-L localize to different subcellular compartments.
(a) Immunofluorescence staining of endogenous ZAP in Huh7 WT and ZAP KO cells. The scale bar represents 20 µm. (b) Subcellular fractionation of ZAP WT and CaaX mutant isoforms after expression in Huh7 ZAP KO cells. For (a, b) representative micrographs and Western blots of three independent experiments with similar results are shown.
Supplementary Figure 4 ZAP-L co-localizes with endolysosomes.
(a, b) Confocal immunofluorescence microscopy of ZAP and (a) Rab5 or (b) Rab7 after co-expression of mCherry-Rab5 or mCherry-Rab7 and tag-less ZAP-S, ZAP-L, or their CaaX mutants in Huh7 ZAP KO cells. The scale bar represents 5 µm. Representative micrographs of three independent experiments with similar results are shown.
Supplementary Figure 5 ZAP-L does not co-localize with the endoplasmic reticulum, peroxisomes or mitochondria.
(a) Confocal immunofluorescence microscopy of ZAP and the endoplasmic reticulum marker Sec61β. Representative micrographs of three independent experiments with similar results are shown. (b) Quantification of co-localization of ZAP-positive and Sec61β-positive pixels. The correlation coefficients (Pearson’s r) of 10 cells were analyzed using the Fiji Coloc 2 plugin. Error bars show mean ± SD. (c) Membrane flotation assay and sedimentation of ZAP-L, ZAP-S, and the endoplasmic reticulum marker calnexin in Huh7 WT cells treated with or without 0.25 µg/ml RIG-I ligand for 24 h. A representative Western blot of two independently performed experiments with similar results is shown. (d, e) Confocal immunofluorescence microscopy of ZAP and (d) the peroxisomal marker PTS1 and (e) the mitochondrial marker COX8 after co-expression of mCherry-tagged PTS1 and COX8 together with tag-less ZAP-L. The scale bar represents 5 µm. (d, e) Representative micrographs of three independent experiments with similar results are shown.
Supplementary Figure 6 ZAP-L targets alphavirus RNA at viral replication sites.
(a) Confocal immunofluorescence microscopy of endogenous ZAP, SINV dsRNA, and E-cadherin in Huh7 WT cells upon infection with SINV Toto (MOI = 1; 6 h post-infection). The scale bar represents 5 µm. (b) Confocal immunofluorescence microscopy of endogenous ZAP, SINV dsRNA, and G3BP1 in Huh7 WT cells upon infection with SINV Toto (MOI = 1; 6 h post-infection). The scale bar represents 5 µm. (a, b) Representative micrographs of three independent experiments with similar results are shown. (c) Replication activity of a Semliki Forest virus (SFV) luciferase replicon in doxycycline-inducible HEK 293 T cells expressing ZAP-S WT, ZAP-L WT, or their respective CaaX motif mutants. A representative experiment with replicates (n = 3) of three independently performed experiments with similar results is shown. Symbols show mean ± SD.
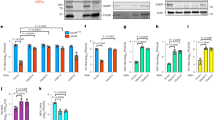
Supplementary Figure 7 ZAP-S, but not ZAP-L, suppresses IFN.
(a) Western blot of ZAP isoform expression and (b) IFNB and IFNL3 mRNA expression in HEK 293 cells after isoform-specific knockdown of ZAP and stimulation with 1 µg/ml RIG-I ligand for 30 h. (a, b) A representative experiment with replicates (n = 3) of three independently performed experiments with similar results is shown. Bars show mean ± SD. (c) IL6 and TNFA mRNA expression in Huh7 cells upon isoform-specific knockdown of ZAP and stimulation with 0.5 µg/ml RIG-I ligand for 42 h. A representative experiment with replicates (n = 3) of two independently performed experiments with similar results is shown. Bars show mean ± SD. (d) ZAP RNA-IP performed in Huh7 ZAP KO cells after expression of FLAG-tagged ZAP-S, FLAG-tagged ZAP-L and FLAG-empty vector (EV) control, and stimulation with 0.2 µg/ml RIG-I ligand for 24 h. A representative experiment of two independently performed experiments with similar results is shown. (e) Expression of IFNB and IFNL3 mRNA and (f) Western blot of IRF1 protein expression in Huh7 WT and ZAP KO cells 48 h after overexpression of IRF1. (e, f) A representative experiment with replicates (n = 3) of three independently performed experiments with similar results is shown. Bars show mean ± SD. Data were analyzed using (b, c) one-way ANOVA with Tukey’s post-test or (e) unpaired two-tailed Student’s t-test. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
Supplementary Figure 8 Model of ZAP-mediated post-transcriptional regulation during innate antiviral immunity.
Constitutively expressed ZAP-L localizes to endolysosomes and sites of Sindbis virus replication at the plasma membrane to target viral RNA (vRNA) and inhibit viral replication. Escaping vRNA triggers expression and secretion of type I and III IFNs, which engage and signal through the IFN receptors. This induces CSTF2-mediated alternative polyadenylation and alternative last exon usage of ZC3HAV1 and expression of ZAP-S, which then binds to IFN mRNA in the cytoplasm to mediate resolution of the antiviral innate immune response.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8 and Tables 1–3.
Rights and permissions
About this article
Cite this article
Schwerk, J., Soveg, F.W., Ryan, A.P. et al. RNA-binding protein isoforms ZAP-S and ZAP-L have distinct antiviral and immune resolution functions. Nat Immunol 20, 1610–1620 (2019). https://doi.org/10.1038/s41590-019-0527-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-019-0527-6
This article is cited by
-
RNAi-based drug design: considerations and future directions
Nature Reviews Drug Discovery (2024)
-
Evolution of the Major Components of Innate Immunity in Animals
Journal of Molecular Evolution (2024)
-
The nexus between RNA-binding proteins and their effectors
Nature Reviews Genetics (2023)
-
Intracellular mono-ADP-ribosyltransferases at the host–virus interphase
Cellular and Molecular Life Sciences (2022)
-
The short isoform of the host antiviral protein ZAP acts as an inhibitor of SARS-CoV-2 programmed ribosomal frameshifting
Nature Communications (2021)