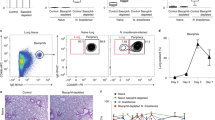
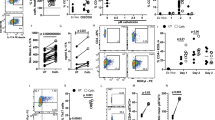
Abstract
Basophils are evolutionarily conserved in vertebrates, despite their small numbers and short life span, suggesting that they have beneficial roles in maintaining health. However, these roles are not fully defined. Here we demonstrate that basophil-deficient mice exhibit reduced bacterial clearance and increased morbidity and mortality in the cecal ligation and puncture (CLP) model of sepsis. Among the several proinflammatory mediators that we measured, tumor necrosis factor (TNF) was the only cytokine that was significantly reduced in basophil-deficient mice after CLP. In accordance with that observation, we found that mice with genetic ablation of Tnf in basophils exhibited reduced systemic concentrations of TNF during endotoxemia. Moreover, after CLP, mice whose basophils could not produce TNF, exhibited reduced neutrophil and macrophage TNF production and effector functions, reduced bacterial clearance, and increased mortality. Taken together, our results show that basophils can enhance the innate immune response to bacterial infection and help prevent sepsis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The authors declare that the main data supporting the findings of this study are available within the article and its supplementary figures. Additional data are available from the corresponding authors upon request.
References
Ehrlich, P. Ueber die specifischen Granulationen des Blutes. Archiv fuer Anatomie und Physiologie: Physiologische Abteilung 571–579, Archiv (1879).
Canfield, P. J. Comparative cell morphology in the peripheral blood film from exotic and native animals. Aust. Vet. J. 76, 793–800 (1998).
Eberle, J. U. & Voehringer, D. Role of basophils in protective immunity to parasitic infections. Semin. Immunopathol. 38, 605–613 (2016).
Karasuyama, H. & Yamanishi, Y. Basophils have emerged as a key player in immunity. Curr. Opin. Immunol. 31, 1–7 (2014).
Mukai, K., Karasuyama, H., Kabashima, K., Kubo, M. & Galli, S. J. Differences in the importance of mast cells, basophils, IgE, and IgG versus that of CD4+ T cells and ILC2 cells in primary and secondary immunity to Strongyloides venezuelensis. Infect. Immun. 85, e00053-17 (2017).
Reitz, M. et al. Mucosal mast cells are indispensable for the timely termination of Strongyloides ratti infection. Mucosal Immunol 10, 481–492 (2017).
Siracusa, M. C., Kim, B. S., Spergel, J. M. & Artis, D. Basophils and allergic inflammation. J. Allergy Clin. Immunol. 132, 789–801 (2013).
Hamilton, R. G., MacGlashan, D. W. Jr & Saini, S. S. IgE antibody-specific activity in human allergic disease. Immunol. Res. 47, 273–284 (2010).
Marone, G., Borriello, F., Varricchi, G., Genovese, A. & Granata, F. Basophils: historical reflections and perspectives. Chem. Immunol. Allergy 100, 172–192 (2014).
Mukai, K. et al. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity 23, 191–202 (2005).
Sato, E. et al. Chronic inflammation of the skin can be induced in IgE transgenic mice by means of a single challenge of multivalent antigen. J. Allergy Clin. Immunol. 111, 143–148 (2003).
Ohnmacht, C. & Voehringer, D. Basophil effector function and homeostasis during helminth infection. Blood 113, 2816–2825 (2009).
Leung, D. Y. et al. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J. Clin. Invest. 92, 1374–1380 (1993).
Yousefi, S. et al. Basophils exhibit antibacterial activity through extracellular trap formation. Allergy 70, 1184–1188 (2015).
Dejager, L., Pinheiro, I., Dejonckheere, E. & Libert, C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 19, 198–208 (2011).
Poli-de-Figueiredo, L. F., Garrido, A. G., Nakagawa, N. & Sannomiya, P. Experimental models of sepsis and their clinical relevance. Shock 30(Suppl 1), 53–59 (2008).
Rittirsch, D., Hoesel, L. M. & Ward, P. A. The disconnect between animal models of sepsis and human sepsis. J. Leukoc. Biol. 81, 137–143 (2007).
Yoshimoto, T. et al. Basophils contribute to TH2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat. Immunol. 10, 706–712 (2009).
Ugajin, T. et al. Basophils preferentially express mouse Mast Cell Protease 11 among the mast cell tryptase family in contrast to mast cells. J. Leukoc. Biol. 86, 1417–1425 (2009).
Sullivan, B. M. et al. Genetic analysis of basophil function in vivo. Nat. Immunol. 12, 527–535 (2011).
Voehringer, D., Liang, H. E. & Locksley, R. M. Homeostasis and effector function of lymphopenia-induced ‘memory-like’ T cells in constitutively T cell-depleted mice. J. Immunol. 180, 4742–4753 (2008).
Gobbetti, T. et al. Nonredundant protective properties of FPR2/ALX in polymicrobial murine sepsis. Proc. Natl Acad. Sci. USA 111, 18685–18690 (2014).
Hoesel, L. M. et al. Harmful and protective roles of neutrophils in sepsis. Shock 24, 40–47 (2005).
Huang, X. et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc. Natl Acad. Sci. USA 106, 6303–6308 (2009).
Flynn, J. L. et al. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2, 561–572 (1995).
Rothe, J. et al. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 364, 798–802 (1993).
Tite, J. P., Dougan, G. & Chatfield, S. N. The involvement of tumor necrosis factor in immunity to Salmonella infection. J. Immunol. 147, 3161–3164 (1991).
Maurer, M. et al. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J. Exp. Med. 188, 2343–2348 (1998).
Grivennikov, S. I. et al. Distinct and nonredundant in vivo functions of TNF produced by T cells and macrophages/neutrophils: protective and deleterious effects. Immunity 22, 93–104 (2005).
Torrero, M. N., Larson, D., Hübner, M. P. & Mitre, E. CD200R surface expression as a marker of murine basophil activation. Clin. Exp. Allergy 39, 361–369 (2009).
Remick, D. G., Newcomb, D. E., Bolgos, G. L. & Call, D. R. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock 13, 110–116 (2000).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Tracey, K. J. et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330, 662–664 (1987).
Männel, D. N. & Echtenacher, B. TNF in the inflammatory response. Chem. Immunol. 74, 141–161 (2000).
Singh, R. K. & Sodhi, A. Effect of TNF priming of murine peritoneal macrophages on their activation to a tumoricidal state. Immunol. Lett. 28, 127–133 (1991).
Wright, H. L., Thomas, H. B., Moots, R. J. & Edwards, S. W. RNA-seq reveals activation of both common and cytokine-specific pathways following neutrophil priming. PLoS ONE 8, e58598 (2013).
Mullen, P. G., Windsor, A. C., Walsh, C. J., Fowler, A. A.3rd & Sugerman, H. J. Tumor necrosis factor-α and interleukin-6 selectively regulate neutrophil function in vitro. J. Surg. Res. 58, 124–130 (1995).
Barton, B. E. & Jackson, J. V. Protective role of interleukin 6 in the lipopolysaccharide-galactosamine septic shock model. Infect. Immun. 61, 1496–1499 (1993).
Barton, B. E., Shortall, J. & Jackson, J. V. Interleukins 6 and 11 protect mice from mortality in a staphylococcal enterotoxin-induced toxic shock model. Infect. Immun. 64, 714–718 (1996).
van der Poll, T. et al. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J. Infect. Dis. 176, 439–444 (1997).
Boomer, J. S. et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306, 2594–2605 (2011).
Eskandari, M. K. et al. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J. Immunol. 148, 2724–2730 (1992).
Echtenacher, B., Falk, W., Männel, D. N. & Krammer, P. H. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J. Immunol. 145, 3762–3766 (1990).
Echtenacher, B., Weigl, K., Lehn, N. & Männel, D. N. Tumor necrosis factor-dependent adhesions as a major protective mechanism early in septic peritonitis in mice. Infect. Immun. 69, 3550–3555 (2001).
Obata, K. et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood 110, 913–920 (2007).
Korosec, P. et al. Basophils, high-affinity IgE receptors, and CCL2 in human anaphylaxis. J. Allergy Clin. Immunol. 140, 750–758.e15 (2017).
Xiao, W. et al. A genomic storm in critically injured humans. J. Exp. Med. 208, 2581–2590 (2011).
Sweeney, T. E., Shidham, A., Wong, H. R. & Khatri, P. A comprehensive time-course-based multicohort analysis of sepsis and sterile inflammation reveals a robust diagnostic gene set. Sci. Transl. Med. 7, 287ra71 (2015).
Piliponsky, A. M. et al. Mast-cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-Kit W-sh/W-sh mice. Am. J. Pathol. 176, 926–938 (2010).
Wada, T. et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J. Clin. Invest. 120, 2867–2875 (2010).
Weighardt, H. et al. Type I IFN modulates host defense and late hyperinflammation in septic peritonitis. J. Immunol. 177, 5623–5630 (2006).
Kasten, K. R. et al. Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through γδ T-cell IL-17 production in a murine model of sepsis. Infect. Immun. 78, 4714–4722 (2010).
Piliponsky, A. M. et al. The chymase mouse mast cell protease 4 degrades TNF, limits inflammation, and promotes survival in a model of sepsis. Am. J. Pathol. 181, 875–886 (2012).
Liu, F. T. et al. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J. Immunol. 124, 2728–2737 (1980).
Acknowledgements
The authors thank R.M. Locksley for providing the Basoph8 mice and members of the Piliponsky and Galli laboratories for helpful discussions. Research in the Piliponsky laboratory was supported by National Institutes of Health (NIH) grant nos. R01 HL113351 and R01 HL141094, and an American Heart Association grant no. 12GRNT9680021. Research in the Galli laboratory was supported by NIH grant nos. R01 CA72074, R37 AI23990, R01 AI070813, and R01 AR067145, and by the Department of Pathology, Stanford University. Research in the Nedospasov laboratory was supported by a grant from the Russian Science Foundation (grant no. 14-50-00060).
Author information
Authors and Affiliations
Contributions
A.M.P., A.K.L., P.T., M.C., N.J.S., L.L.R., K.N., A.L.T., and K.M. performed the experiments. S.A.N. generated the Tnffl/fl mice. A.M.P. and S.J.G. designed the study. A.M.P., S.A.N., H.K., L.L.R., M.T., K.M., and S.J.G. analyzed the data. A.M.P. and S.J.G. wrote the manuscript. All of the authors contributed to the final editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Gating strategy to identify basophils among total peritoneal cells after CLP.
(a-i) Representative flow cytometry plots and frequencies used to identify basophils amongst peritoneal cells obtained from mice 24 h after induction of moderate CLP (50% ligation, one puncture with a 22G needle). (a) Cell population considered for analysis using FSC and SSC. (b) Doublets were removed using FSC-A and FSC-W gating. (c) Dead cells were removed from the analysis using DAPI dead cell stain. (d, e) FcεRIα+ c-Kit- (d) and CD49b+ (e) cells were gated and defined as basophils. (f, g) The same gating strategy was used to identify basophils amongst peritoneal cells obtained from CLP-treated basophil-deficient mice, which shows a significant reduction in the basophil percentage. (h, i) Peritoneal mast cells from naïve mice were used to establish gates for FcεRIα+ and c-Kit- cells. Data are representative of those obtained in the 3 independent experiments performed.
Supplementary Figure 2 Peritoneal neutrophils and macrophages from naïve mice express low levels of Mcpt8 mRNA.
Mcpt8 mRNA expression relative to GAPDH in Gr-1+ CD11b+ neutrophils (Neu) and F4/80+ CD11b+ macrophages (Macs) that were sorted from the peritoneum of naïve Tnffl/fl mice and Basoph8 x Tnffl/fl mice (n = 3 mice/group). Mcpt8 gene expression was assessed by qPCR using FcεRIα+ CD49b+ c-Kit– basophils that were sorted from bone marrow of Tnffl/fl mice as positive controls. Mcpt8 mRNA expression levels in neutrophils and macrophages were normalized against Mcpt8 mRNA expression levels found in bone marrow basophils (Bas). Data are shown as means + SEM with squares representing values for individual experiments. The qPCR data are representative of n = 3 independent experiments performed, each of which gave similar results.
Supplementary Figure 3 TNF enhances bacterial clearance after CLP.
(a-b) Compared with the Tnf+/+ mice, Tnf-/- mice exhibited increased peritoneal cavity (a) and blood (b) colony forming units (CFU) at 24 h after CLP. Data in a and b are shown as means with circles representing values from individual mice (n = 14 and n = 11 for Tnf+/+ mice in a and b, respectively, and n = 7 and n = 6 for Tnf-/- mice in a and b, respectively). P values by Mann-Whitney test.
Supplementary Figure 4 Leukocyte populations in Tnffl/fl mice and Basoph8 × Tnffl/fl mice.
(a-d) Percentages of leukocytes (a) Basophil percentages (FcεRIα+ CD49b+ c-Kit–) in the live-cell population isolated from spleens (Tnffl/fl, n = 7; Basoph8 x Tnffl/fl, n = 9), bone marrow (Tnffl/fl, n = 4; Basoph8 x Tnffl/fl, n = 9), or blood (Tnffl/fl, n = 16; Basoph8 x Tnffl/fl, n = 8), as analyzed by flow cytometry. (b) Neutrophil percentages (Gr-1+ CD11b+) in the live-cell population isolated from spleen (Tnffl/fl, n = 6; Basoph8 x Tnffl/fl, n = 6), bone marrow (Tnffl/fl, n = 6; Basoph8 x Tnffl/fl, n = 7), or peritoneal fluids (Tnffl/fl, n = 7; Basoph8 x Tnffl/fl, n = 10), as analyzed by flow cytometry. (c) Macrophage percentages (F4/80+ CD11b+) in the live-cell population isolated from spleens (Tnffl/fl, n = 7; Basoph8 x Tnffl/fl, n = 10) or peritoneal fluids (Tnffl/fl, n = 3; Basoph8 x Tnffl/fl, n = 10), as analyzed by flow cytometry. Data in a-d are depicted as mean + SEM with circles representing values for individual mice. (d) Mast cell percentages (FcεRIα+ c-Kit+) in the live-cell population isolated from peritoneal fluids (Tnffl/fl, n = 4; Basoph8 x Tnffl/fl, n = 6), as analyzed by flow cytometry. (e-g) Absolute numbers of leukocytes. (e) Blood monocyte (Tnffl/fl, n = 13; Basoph8 x Tnffl/fl, n = 9) and eosinophil (Tnffl/fl, n = 9; Basoph8 x Tnffl/fl, n = 10) numbers. (f) Blood lymphocyte (Tnffl/fl, n = 20; Basoph8 x Tnffl/fl, n = 10) and neutrophil (Tnffl/fl, n = 21; Basoph8 x Tnffl/fl, n = 9) numbers. (g) Blood lymphocyte (Tnffl/fl, n = 9; Basoph8 x Tnffl/fl, n = 9) and neutrophil (Tnffl/fl, n = 10; Basoph8 x Tnffl/fl, n = 8) numbers at 24 h after moderate CLP. Data in e-g are depicted as mean + SEM with circles representing values for individual mice. P values by Mann-Whitney test.
Supplementary Figure 5 Basophil-derived TNF contributes to increased TNF production by peritoneal macrophages during LPS-induced endotoxemia.
Representative flow cytometry plots for TNF positive cells in the macrophage populations obtained from the peritoneal cavity of Tnffl/fl and Basoph8 x Tnffl/fl mice (n = 4/group) 1 h after i.p. injection of LPS (10 μg). Cells were immunostained for TNF in addition to surface F4/80 and CD11b to identify macrophages. Data were obtained from two independent experiments performed, each of which gave similar results.
Supplementary Figure 6 Disruption of Mcpt8 expression levels in Basoph8 mice does not impact the outcome of CLP of moderate severity.
(a, b) Mcpt8 expression levels are significantly reduced or undetectable in basophils from mice with one or two Basoph8 gene copies, respectively. Mcpt8 mRNA (a) and protein (b) expression levels in basophils that were sorted from bone marrow cells harvested from naïve mice (a) or from FcεRIα+ CD49b+ c-Kit– bone marrow-derived cells cultured in the presence of IL-3 (b). The qPCR data in a and western blot analysis in b are representative of 2 independent experiments performed, each of which gave similar results. The Western blot analysis in b was cropped from the original blot shown in Supplementary Fig. 7. (c, d) Basophil-derived TNF-deficient mice with one Basoph8 gene copy (mice with a significant reduction in Mcpt8 expression levels) exhibit similar mortality and morbidity as basophil-derived TNF-deficient mice with two Basoph8 gene copies (mice with a total Mcpt8 deficiency). Survival after moderate CLP in littermate control mice (Tnffl/fl = Basoph8 -/- x Tnffl/fl, n = 15), Basoph8+/- x Tnffl/fl (n = 7) and Basoph8+/+ x Tnffl/fl (n = 7). *P < 0.05 and **P < 0.01 vs. Tnffl/fl mice. P values by Logrank (Mantel-Cox test). (d) Drop in body temperature in Tnffl/fl mice (n = 7), Basoph8± x Tnffl/fl mice (n = 13) and Basoph8+/+ x Tnffl/fl mice (n = 17) at 24 h after CLP. P values by Mann-Whitney test. Data in c were pooled from the n = 3 independent experiments performed, each of which gave similar results. Data in d are shown as means + SEM with circles representing values for individual mice and were pooled from n = 3 independent experiments performed, each of which gave similar results. (e-j) Mice with one Basoph8 gene copy (that is, Basoph8± x Tnf +/+ mice with no Mcpt8 protein expression, but which express basophil-derived TNF) exhibit morbidity rates similar to their littermate controls [Tnf +/+ mice]). (e-j) Drop in body temperature (e), colony forming units (CFU) in peritoneal cavity (f) and blood (g), amount of intraperitoneal TNF (h), and numbers of neutrophils (i) and macrophages (j) in the peritoneal live-cell population analyzed by flow cytometry (Gr-1+ CD11b+ cells for neutrophils and F4/80+ CD11b+ for macrophages) in Basoph8-/- x Tnf+/+ mice and Basoph8± x Tnf+/+ mice at 24 h after CLP. Data are shown as means + SEM with circles representing values for individual mice (n = 23 in e, n = 19 in f, n = 16 in g, n = 14 in h and n = 7 in i and j for Basoph8-/- x Tnf+/+ mice; and n = 9 in e, n = 14 in f, n = 13 in g, n = 7 in h and n = 5 in i and j for Basoph8± x Tnf+/+ mice. Data were pooled from n = 3 independent experiments performed, each of which gave similar results.
Supplementary Figure 7 Full scans of blots used in Supplementary Fig. 6.
Blots show Mcpt8 (a) and GAPDH (b) expression levels in basophils from mice with one or two Basoph8 gene copies, respectively. Mcpt8 and GAPDH protein expression levels in increasing number of basophils that were sorted from FcεRIα+ CD49b+ c-Kit– bone marrow-derived cells cultured in the presence of IL-3. Blots are representative of 2 independent experiments performed, each of which gave similar results.
Supplementary Figure 8 Neutrophils, macrophages and mast cells from Basoph8 × Tnffl/fl mice express negligible YFP and Mcpt8 mRNA after CLP.
(a) Representative flow cytometry plots and cell frequencies in the peritoneal neutrophil, macrophage, mast cell and basophil populations obtained from Tnffl/fl mice (n = 3) and Basoph8± x Tnffl/fl mice (n = 3) at 24 h after moderate CLP. Cells were immunostained for surface Gr-1 and CD11b for peritoneal neutrophils; with F4/80 and CD11b for macrophages; and with FcεRIα, c-Kit and CD49b for mast cells (FcεRIα+ c-Kit+) and basophils (FcεRIα+ CD49b+). (b) The specificity of Mcpt8 gene expression as assessed by single cell PCR detection in peritoneal neutrophils (Neu), macrophages (Macs), mast cells (MCs), and BM basophils (BMBas) obtained from Tnffl/fl mice and Basoph8± x Tnffl/fl mice at 24 h after moderate CLP. The numbers above the bar graphs indicate the number of cells analyzed. Results are expressed as % of cells expressing transcripts for the PCR products analyzed/cell type; results were pooled from n = 3 sorting experiments.
Supplementary Figure 9 TNF ablation in basophils does not affect ROS production and phagocytosis in neutrophils and macrophages ex vivo.
Neutrophil ROS production (a) and macrophage phagocytosis (b). Bone marrow neutrophils were loaded for 30 min at 37 °C with 1 μM of CM-H2DCFDA, which is a general oxidative stress indicator, and then activated with 100 ng/ml of PMA. Peritoneal macrophages were incubated with opsonized FITC-labeled E. coli (K-12 strain) bioparticles for 10 min at 37 °C. Cells were stained for neutrophil and macrophage-specific surface markers and flow cytometry analysis was performed immediately thereafter. Data in a and b are shown as means + SEM with squares showing values for individual mice (n = 4 for Tnffl/fl mice in a and b, and n = 3 and n = 4 for Basoph8 x Tnffl/fl mice in a and b, respectively). Data were pooled from n = 2 independent experiments.
Supplementary Figure 10 Intracellular staining for IL-6 in myeloid cells during CLP.
Flow cytometry plots and frequencies (a) and mean fluorescence intensities (b) for IL-6 positive cells in the peritoneal neutrophil and macrophage populations obtained from Tnffl/fl mice and Basoph8 x Tnffl/fl mice (n = 6 mice/group) at 24 h after CLP and then incubated ex vivo for 4 h in the presence of GolgiPlug. Cells were immunostained for IL-6 in addition to surface Gr-1 and CD11b to identify peritoneal neutrophils or surface F4/80 and CD11b to identify macrophages. Data in a are from one representative experiment of the n = 2 independent experiments performed, each of which gave similar results. The data in b were pooled from n = 2 independent experiments that were performed. Data are shown as means + SEM with squares showing values for cells obtained from individual mice (n = 6/group). P values by Mann-Whitney test.
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–10 and Supplementary Table 1. Association between FCER1A expression in whole blood and clinical attributes in trauma patients at 7 d after severe injury. The dataset was obtained from 167 severely injured patients and compared with 35 healthy individuals (ages 16–55) (http://web.mgh.harvard.edu/TRT/).
Rights and permissions
About this article
Cite this article
Piliponsky, A.M., Shubin, N.J., Lahiri, A.K. et al. Basophil-derived tumor necrosis factor can enhance survival in a sepsis model in mice. Nat Immunol 20, 129–140 (2019). https://doi.org/10.1038/s41590-018-0288-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-018-0288-7
This article is cited by
-
Basophils absence predicts poor prognosis and indicates immunosuppression of patients in intensive care units
Scientific Reports (2023)
-
Association of peripheral basophils with tumor M2 macrophage infiltration and outcomes of the anti-PD-1 inhibitor plus chemotherapy combination in advanced gastric cancer
Journal of Translational Medicine (2022)
-
A Myb enhancer-guided analysis of basophil and mast cell differentiation
Nature Communications (2022)
-
Identification and characterization of neutrophil heterogeneity in sepsis
Critical Care (2021)
-
Skeletal muscle fibers play a functional role in host defense during sepsis in mice
Scientific Reports (2021)