Abstract
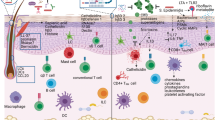
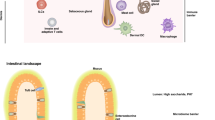
The skin provides both a physical barrier and an immunologic barrier to external threats. The protective machinery of the skin has evolved to provide situation-specific responses to eliminate pathogens and to provide protection against physical dangers. Dysregulation of this machinery can give rise to the initiation and propagation of inflammatory loops in the epithelial microenvironment that result in inflammatory skin diseases in susceptible people. A defective barrier and microbial dysbiosis drive an interleukin 4 (IL-4) loop that underlies atopic dermatitis, while in psoriasis, disordered keratinocyte signaling and predisposition to type 17 responses drive a pathogenic IL-17 loop. Here we discuss the pathogenesis of atopic dermatitis and psoriasis in terms of the epithelial immune microenvironment—the microbiota, keratinocytes and sensory nerves—and the resulting inflammatory loops.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dainichi, T., Hanakawa, S. & Kabashima, K. Classification of inflammatory skin diseases: a proposal based on the disorders of the three-layered defense systems, barrier, innate immunity and acquired immunity. J. Dermatol. Sci. 76, 81–89 (2014).
Bjerke, J. R. In situ characterization and counting of mononuclear cells in lesions of different clinical forms of psoriasis. Acta Derm. Venereol. 62, 93–100 (1982).
Ellis, C. N. et al. Cyclosporine improves psoriasis in a double-blind study. J. Am. Med. Assoc. 256, 3110–3116 (1986).
Gottlieb, S. L. et al. Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat. Med. 1, 442–447 (1995).
Hammar, H., Gu, S. Q., Johannesson, A., Sundkvist, K. G. & Biberfeld, P. Subpopulations of mononuclear cells in microscopic lesions of psoriatic patients. Selective accumulation of suppressor/cytotoxic T cells in epidermis during the evolution of the lesion. J. Invest. Dermatol. 83, 416–420 (1984).
Mueller, W. & Herrmann, B. Cyclosporin A for psoriasis. N. Engl. J. Med. 301, 555 (1979).
Faulds, D., Goa, K. L. & Benfield, P. Cyclosporin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs 45, 953–1040 (1993).
Kabashima, K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J. Dermatol. Sci. 70, 3–11 (2013).
Eichenfield, L. F. et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J. Am. Acad. Dermatol. 70, 338–351 (2014).
Furue, M. et al. Prevalence of dermatological disorders in Japan: a nationwide, cross-sectional, seasonal, multicenter, hospital-based study. J. Dermatol. 38, 310–320 (2011).
Simpson, E. L. et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N. Engl. J. Med. 375, 2335–2348 (2016).
Kim, B. S. et al. Basophils promote innate lymphoid cell responses in inflamed skin. J. Immunol. 193, 3717–3725 (2014).
Novak, N. & Bieber, T. Allergic and nonallergic forms of atopic diseases. J. Allergy Clin. Immunol. 112, 252–262 (2003).
Horimukai, K. et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J. Allergy Clin. Immunol. 134, 824–830 (2014).
Oldhoff, J. M. et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy 60, 693–696 (2005).
Paller, A. S., Kabashima, K. & Bieber, T. Therapeutic pipeline for atopic dermatitis: End of the drought? J. Allergy Clin. Immunol. 140, 633–643 (2017).
Guttman-Yassky, E. et al. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J. Am. Acad. Dermatol. 78, 872–881 (2018).
Perera, G. K., Di Meglio, P. & Nestle, F. O. Psoriasis. Annu. Rev. Pathol. 7, 385–422 (2012).
Lowes, M. A., Suárez-Fariñas, M. & Krueger, J. G. Immunology of psoriasis. Annu. Rev. Immunol. 32, 227–255 (2014).
Kim, J. & Krueger, J. G. Highly effective new treatments for psoriasis target the IL-23/type 17 T cell autoimmune axis. Annu. Rev. Med. 68, 255–269 (2017).
Lowes, M. A. et al. Increase in TNF-α and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc. Natl. Acad. Sci. USA 102, 19057–19062 (2005).
Serbina, N. V., Salazar-Mather, T. P., Biron, C. A., Kuziel, W. A. & Pamer, E. G. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19, 59–70 (2003).
Zaba, L. C. et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J. Exp. Med. 204, 3183–3194 (2007).
Zaba, L. C. et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J. Invest. Dermatol. 129, 79–88 (2009).
Zaba, L. C., Krueger, J. G. & Lowes, M. A. Resident and “inflammatory” dendritic cells in human skin. J. Invest. Dermatol. 129, 302–308 (2009).
Chiricozzi, A. et al. Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J. Invest. Dermatol. 131, 677–687 (2011).
Papp, K. A. et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N. Engl. J. Med. 376, 1551–1560 (2017).
Kolls, J. K. & Lindén, A. Interleukin-17 family members and inflammation. Immunity 21, 467–476 (2004).
Griffiths, C. E. et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 386, 541–551 (2015).
Krueger, J. G. et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J. Allergy Clin. Immunol. 130, 145–154 (2012).
Langley, R. G. et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N. Engl. J. Med. 371, 326–338 (2014).
Lebwohl, M. et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N. Engl. J. Med. 373, 1318–1328 (2015).
Lin, A. M. et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 187, 490–500 (2011).
Teunissen, M. B. M. et al. Composition of innate lymphoid cell subsets in the human skin: enrichment of NCR+ ILC3 in lesional skin and blood of psoriasis patients. J. Invest. Dermatol. 134, 2351–2360 (2014).
Villanova, F. et al. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J. Invest. Dermatol. 134, 984–991 (2014).
Fujita, H. The role of IL-22 and Th22 cells in human skin diseases. J. Dermatol. Sci. 72, 3–8 (2013).
Tsai, Y. C. & Tsai, T. F. Anti-interleukin and interleukin therapies for psoriasis: current evidence and clinical usefulness. Ther. Adv. Musculoskelet. Dis. 9, 277–294 (2017).
Shibata, S. et al. IL-27 activates Th1-mediated responses in imiquimod-induced psoriasis-like skin lesions. J. Invest. Dermatol. 133, 479–488 (2013).
Chen, W. et al. Decreased expression of IL-27 in moderate-to-severe psoriasis and its anti-inflammation role in imiquimod-induced psoriasis-like mouse model. J. Dermatol. Sci. 85, 115–123 (2017).
Egawa, G. & Kabashima, K. Multifactorial skin barrier deficiency and atopic dermatitis: Essential topics to prevent the atopic march. J. Allergy Clin. Immunol. 138, 350–358 (2016).
Tsunemi, Y. et al. Interleukin-13 gene polymorphism G4257A is associated with atopic dermatitis in Japanese patients. J. Dermatol. Sci. 30, 100–107 (2002).
Howard, T. D. et al. Identification and association of polymorphisms in the interleukin-13 gene with asthma and atopy in a Dutch population. Am. J. Respir. Cell Mol. Biol. 25, 377–384 (2001).
Palmer, C. N. et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 38, 441–446 (2006).
Palmer, C. N. et al. Filaggrin null mutations are associated with increased asthma severity in children and young adults. J. Allergy Clin. Immunol. 120, 64–68 (2007).
Esparza-Gordillo, J. et al. A common variant on chromosome 11q13 is associated with atopic dermatitis. Nat. Genet. 41, 596–601 (2009).
Paternoster, L. et al. Meta-analysis of genome-wide association studies identifies three new risk loci for atopic dermatitis. Nat. Genet. 44, 187–192 (2011).
Ellinghaus, D. et al. High-density genotyping study identifies four new susceptibility loci for atopic dermatitis. Nat. Genet. 45, 808–812 (2013).
Hirota, T. et al. Genome-wide association study identifies eight new susceptibility loci for atopic dermatitis in the Japanese population. Nat. Genet. 44, 1222–1226 (2012).
Weidinger, S. et al. A genome-wide association study of atopic dermatitis identifies loci with overlapping effects on asthma and psoriasis. Hum. Mol. Genet. 22, 4841–4856 (2013).
Tamari, M. & Hirota, T. Genome-wide association studies of atopic dermatitis. J. Dermatol. 41, 213–220 (2014).
Anbunathan, H. & Bowcock, A. M. The molecular revolution in cutaneous biology: the era of genome-wide association studies and statistical, big data, and computational topics. J. Invest. Dermatol. 137, e113–e118 (2017).
Armstrong, A. W., Harskamp, C. T. & Armstrong, E. J. Psoriasis and metabolic syndrome: a systematic review and meta-analysis of observational studies. J. Am. Acad. Dermatol. 68, 654–662 (2013).
England, B. R., Thiele, G. M., Anderson, D. R. & Mikuls, T. R. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. Br. Med. J. 361, k1036 (2018).
Ha, C., Magowan, S., Accortt, N. A., Chen, J. & Stone, C. D. Risk of arterial thrombotic events in inflammatory bowel disease. Am. J. Gastroenterol. 104, 1445–1451 (2009).
Atzeni, F. et al. Behçet’s disease and cardiovascular involvement. Lupus 14, 723–726 (2005).
Jordan, C. T. et al. Rare and common variants in CARD14, encoding an epidermal regulator of NF-κB, in psoriasis. Am. J. Hum. Genet. 90, 796–808 (2012).
Marrakchi, S. et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N. Engl. J. Med. 365, 620–628 (2011).
Tsoi, L. C. et al. Enhanced meta-analysis and replication studies identify five new psoriasis susceptibility loci. Nat. Commun. 6, 7001 (2015).
Wohn, C. et al. Langerinneg conventional dendritic cells produce IL-23 to drive psoriatic plaque formation in mice. Proc. Natl. Acad. Sci. USA 110, 10723–10728 (2013).
Nakamura, Y. et al. Staphylococcus δ-toxin induces allergic skin disease by activating mast cells. Nature 503, 397–401 (2013).
Van Dyken, S. J. et al. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and γδ T cells. Immunity 40, 414–424 (2014).
Li, M. et al. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl. Acad. Sci. USA 103, 11736–11741 (2006).
Nakajima, S. et al. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J. Allergy Clin. Immunol. 129, 1048–1055 (2012).
Leyva-Castillo, J. M., Hener, P., Jiang, H. & Li, M. TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J. Invest. Dermatol. 133, 154–163 (2013).
Sokol, C. L., Barton, G. M., Farr, A. G. & Medzhitov, R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat. Immunol. 9, 310–318 (2008).
Ohnmacht, C. et al. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 33, 364–374 (2010).
Tang, H. et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat. Immunol. 11, 608–617 (2010).
Halim, T. Y., Krauss, R. H., Sun, A. C. & Takei, F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity 36, 451–463 (2012).
Motomura, Y. et al. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity 40, 758–771 (2014).
Hammad, H. et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15, 410–416 (2009).
Lambrecht, B. N. & Hammad, H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat. Immunol. 18, 1076–1083 (2017).
Nowarski, R., Jackson, R. & Flavell, R. A. The stromal intervention: regulation of immunity and inflammation at the epithelial-mesenchymal barrier. Cell 168, 362–375 (2017).
van der Fits, L. et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182, 5836–5845 (2009).
Guttman-Yassky, E. et al. Major differences in inflammatory dendritic cells and their products distinguish atopic dermatitis from psoriasis. J. Allergy Clin. Immunol. 119, 1210–1217 (2007).
Chan, J. R. et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J. Exp. Med. 203, 2577–2587 (2006).
Matsumoto, R. et al. Epithelial TRAF6 drives IL-17-mediated psoriatic inflammation. JCI Insight 3, e121175 (2018).
Zhu, H. et al. RIG-I antiviral signaling drives interleukin-23 production and psoriasis-like skin disease. EMBO Mol. Med. 9, 589–604 (2017).
Yoshida, K. et al. Distinct behavior of human Langerhans cells and inflammatory dendritic epidermal cells at tight junctions in patients with atopic dermatitis. J. Allergy Clin. Immunol. 134, 856–864 (2014).
Yoshiki, R. et al. IL-23 from Langerhans cells is required for the development of imiquimod-induced psoriasis-like dermatitis by induction of IL-17A-producing γδ T cells. J. Invest. Dermatol. 134, 1912–1921 (2014).
Belkaid, Y. & Tamoutounour, S. The influence of skin microorganisms on cutaneous immunity. Nat. Rev. Immunol. 16, 353–366 (2016).
Williams, M. R., Nakatsuji, T. & Gallo, R. L. Staphylococcus aureus: master manipulator of the skin. Cell Host Microbe 22, 579–581 (2017).
Lee, H. M. et al. Innate immune responses to Mycobacterium ulcerans via toll-like receptors and dectin-1 in human keratinocytes. Cell. Microbiol. 11, 678–692 (2009).
Kashem, S. W. et al. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity 43, 515–526 (2015).
Grice, E. A. & Segre, J. A. The skin microbiome. Nat. Rev. Microbiol. 9, 244–253 (2011).
Kong, H. H. et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 22, 850–859 (2012).
Byrd, A.L. et al. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci. Transl. Med. 9, eaal4651 (2017).
Langan, E. A. et al. The role of the microbiome in psoriasis: moving from disease description to treatment selection? Br. J. Dermatol. 178, 1020–1027 (2018).
Ruiz-Romeu, E. et al. Microbe-dependent induction of IL-9 by CLA+ T cells in psoriasis and relationship with IL-17A. J. Invest. Dermatol. 138, 580–587 (2018).
Loesche, M. A. et al. Longitudinal study of the psoriasis-associated skin microbiome during therapy with ustekinumab in a randomized phase 3b clinical trial. J. Invest. Dermatol. 138, 1973–1981 (2018).
Alekseyenko, A. V. et al. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome 1, 31 (2013).
Gao, Z., Tseng, C. H., Strober, B. E., Pei, Z. & Blaser, M. J. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS One 3, e2719 (2008).
Amaya, M. et al. Molecular analysis of Malassezia microflora in the lesional skin of psoriasis patients. J. Dermatol. 34, 619–624 (2007).
Takemoto, A., Cho, O., Morohoshi, Y., Sugita, T. & Muto, M. Molecular characterization of the skin fungal microbiome in patients with psoriasis. J. Dermatol. 42, 166–170 (2015).
van den Oord, R. A. & Sheikh, A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. Br. Med. J. 339, b2433 (2009).
Rodriguez, E. et al. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J. Allergy Clin. Immunol. 123, 1361–1370 (2009).
Weidinger, S. et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J. Allergy Clin. Immunol. 118, 214–219 (2006).
Howell, M. D. et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 120, 150–155 (2007).
Chavanas, S. et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat. Genet. 25, 141–142 (2000).
De Benedetto, A. et al. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 127, 773–786 (2011).
Samuelov, L. et al. Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting. Nat. Genet. 45, 1244–1248 (2013).
McAleer, M. A. et al. Severe dermatitis, multiple allergies, and metabolic wasting syndrome caused by a novel mutation in the N-terminal plakin domain of desmoplakin. J. Allergy Clin. Immunol. 136, 1268–1276 (2015).
Kubo, A., Nagao, K. & Amagai, M. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J. Clin. Invest. 122, 440–447 (2012).
Franzke, C. W. et al. Epidermal ADAM17 maintains the skin barrier by regulating EGFR ligand-dependent terminal keratinocyte differentiation. J. Exp. Med. 209, 1105–1119 (2012).
Murthy, A. et al. Notch activation by the metalloproteinase ADAM17 regulates myeloproliferation and atopic barrier immunity by suppressing epithelial cytokine synthesis. Immunity 36, 105–119 (2012).
Kobayashi, T. et al. Dysbiosis and Staphylococcus aureus colonization drives inflammation in atopic dermatitis. Immunity 42, 756–766 (2015).
Tagami, H. & Yoshikuni, K. Interrelationship between water-barrier and reservoir functions of pathologic stratum corneum. Arch. Dermatol. 121, 642–645 (1985).
Takahashi, H., Tsuji, H., Minami-Hori, M., Miyauchi, Y. & Iizuka, H. Defective barrier function accompanied by structural changes of psoriatic stratum corneum. J. Dermatol. 41, 144–148 (2014).
Yuki, T., Tobiishi, M., Kusaka-Kikushima, A., Ota, Y. & Tokura, Y. Impaired tight junctions in atopic dermatitis skin and in a skin-equivalent model treated with interleukin-17. PLoS One 11, e0161759 (2016).
Gutowska-Owsiak, D. et al. IL-17 downregulates filaggrin and affects keratinocyte expression of genes associated with cellular adhesion. Exp. Dermatol. 21, 104–110 (2012).
Doebel, T., Voisin, B. & Nagao, K. Langerhans cells - the macrophage in dendritic cell clothing. Trends Immunol. 38, 817–828 (2017).
Nakajima, K. et al. Barrier abnormality due to ceramide deficiency leads to psoriasiform inflammation in a mouse model. J. Invest. Dermatol. 133, 2555–2565 (2013).
Dumortier, A. et al. Atopic dermatitis-like disease and associated lethal myeloproliferative disorder arise from loss of Notch signaling in the murine skin. PLoS One 5, e9258 (2010).
Volpe, E. et al. Thymic stromal lymphopoietin links keratinocytes and dendritic cell-derived IL-23 in patients with psoriasis. J. Allergy Clin. Immunol. 134, 373–381 (2014).
Li, M. et al. Induction of thymic stromal lymphopoietin expression in keratinocytes is necessary for generating an atopic dermatitis upon application of the active vitamin D3 analogue MC903 on mouse skin. J. Invest. Dermatol. 129, 498–502 (2009).
Briot, A. et al. Par2 inactivation inhibits early production of TSLP, but not cutaneous inflammation, in Netherton syndrome adult mouse model. J. Invest. Dermatol. 130, 2736–2742 (2010).
Hidaka, T. et al. The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat. Immunol. 18, 64–73 (2017).
Junghans, V., Jung, T. & Neumann, C. Human keratinocytes constitutively express IL-4 receptor molecules and respond to IL-4 with an increase in B7/BB1 expression. Exp. Dermatol. 5, 316–324 (1996).
Lambert, S., Swindell, W. R., Tsoi, L. C., Stoll, S. W. & Elder, J. T. Dual role of Act1 in keratinocyte differentiation and host defense: TRAF3IP2 silencing alters keratinocyte differentiation and inhibits IL-17 responses. J. Invest. Dermatol. 137, 1501–1511 (2017).
Ha, H. L. et al. IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms. Proc. Natl. Acad. Sci. USA 111, E3422–E3431 (2014).
Wu, L. et al. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J. Exp. Med. 212, 1571–1587 (2015).
Li, B. et al. Transcriptome analysis of psoriasis in a large case-control sample: RNA-seq provides insights into disease mechanisms. J. Invest. Dermatol. 134, 1828–1838 (2014).
Kumari, S. et al. Tumor necrosis factor receptor signaling in keratinocytes triggers interleukin-24-dependent psoriasis-like skin inflammation in mice. Immunity 39, 899–911 (2013).
Grinberg-Bleyer, Y. et al. Cutting edge: NF-κB p65 and c-Rel control epidermal development and immune homeostasis in the skin. J. Immunol. 194, 2472–2476 (2015).
Rabeony, H. et al. IMQ-induced skin inflammation in mice is dependent on IL-1R1 and MyD88 signaling but independent of the NLRP3 inflammasome. Eur. J. Immunol. 45, 2847–2857 (2015).
Yamamoto, K. et al. The role of group IIF-secreted phospholipase A2 in epidermal homeostasis and hyperplasia. J. Exp. Med. 212, 1901–1919 (2015).
Taylor, P. R. et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2. Nat. Immunol. 15, 143–151 (2014).
Ganguly, D. et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 206, 1983–1994 (2009).
Lande, R. et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449, 564–569 (2007).
Zhang, L. J. et al. Antimicrobial oeptide LL37 and MAVS signaling drive interferon-β production by epidermal keratinocytes during skin injury. Immunity 45, 119–130 (2016).
Oetjen, L. K. et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 171, 217–228 (2017).
Wilson, S. R. et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 155, 285–295 (2013).
Dillon, S. R. et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 5, 752–760 (2004).
Cevikbas, F. et al. A sensory neuron–expressed IL-31 receptor mediates T helper cell–dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 133, 448–460 (2014).
Sonkoly, E. et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 117, 411–417 (2006).
Cornelissen, C. et al. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J. Allergy Clin. Immunol. 129, 426–433 (2012).
Kasraie, S., Niebuhr, M. & Werfel, T. Interleukin (IL)-31 activates signal transducer and activator of transcription (STAT)-1, STAT-5 and extracellular signal-regulated kinase 1/2 and down-regulates IL-12p40 production in activated human macrophages. Allergy 68, 739–747 (2013).
Ruzicka, T. et al. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N. Engl. J. Med. 376, 826–835 (2017).
Cowden, J. M., Zhang, M., Dunford, P. J. & Thurmond, R. L. The histamine H4 receptor mediates inflammation and pruritus in Th2-dependent dermal inflammation. J. Invest. Dermatol. 130, 1023–1033 (2010).
Mollanazar, N. K., Smith, P. K. & Yosipovitch, G. Mediators of chronic pruritus in atopic dermatitis: getting the itch out? Clin. Rev. Allergy Immunol. 51, 263–292 (2016).
Kido-Nakahara, M., Furue, M., Ulzii, D. & Nakahara, T. Itch in atopic dermatitis. Immunol. Allergy Clin. North Am. 37, 113–122 (2017).
Moriyama, S. et al. β2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science 359, 1056–1061 (2018).
Chiu, I. M. et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501, 52–57 (2013).
LaMotte, R. H., Dong, X. & Ringkamp, M. Sensory neurons and circuits mediating itch. Nat. Rev. Neurosci. 15, 19–31 (2014).
Riol-Blanco, L. et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510, 157–161 (2014).
Joseph, T., Kurian, J., Warwick, D. J. & Friedmann, P. S. Unilateral remission of psoriasis following traumatic nerve palsy. Br. J. Dermatol. 152, 185–186 (2005).
Zanchi, M. et al. Botulinum toxin type-A for the treatment of inverse psoriasis. J. Eur. Acad. Dermatol. Venereol. 22, 431–436 (2008).
Nash, M. S. Known and plausible modulators of depressed immune functions following spinal cord injuries. J. Spinal Cord Med. 23, 111–120 (2000).
Rubin-Asher, D., Zeilig, G., Klieger, M., Adunsky, A. & Weingarden, H. Dermatological findings following acute traumatic spinal cord injury. Spinal Cord 43, 175–178 (2005).
Maruyama, K. et al. Nociceptors boost the resolution of fungal osteoinflammation via the TRP channel-CGRP-Jdp2 axis. Cell Reports 19, 2730–2742 (2017).
Ray-Jones, H., Eyre, S., Barton, A. & Warren, R. B. One SNP at a time: moving beyond GWAS in psoriasis. J. Invest. Dermatol. 136, 567–573 (2016).
Bissonnette, R. et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br. J. Dermatol. 175, 902–911 (2016).
Nakagawa, H., Nemoto, O., Igarashi, A. & Nagata, T. Efficacy and safety of topical JTE-052, a Janus kinase inhibitor, in Japanese adult patients with moderate-to-severe atopic dermatitis: a phase II, multicentre, randomized, vehicle-controlled clinical study. Br. J. Dermatol. 178, 424–432 (2018).
Bissonnette, R. et al. Tofacitinib withdrawal and retreatment in moderate-to-severe chronic plaque psoriasis: a randomized controlled trial. Br. J. Dermatol. 172, 1395–1406 (2015).
Bachelez, H. et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet 386, 552–561 (2015).
Papp, K. A. et al. A randomized phase 2b trial of baricitinib, an oral Janus kinase (JAK) 1/JAK2 inhibitor, in patients with moderate-to-severe psoriasis. Br. J. Dermatol. 174, 1266–1276 (2016).
Papp, K. et al. A phase 2a randomized, double-blind, placebo-controlled, sequential dose-escalation study to evaluate the efficacy and safety of ASP015K, a novel Janus kinase inhibitor, in patients with moderate-to-severe psoriasis. Br. J. Dermatol. 173, 767–776 (2015).
Punwani, N. et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J. Am. Acad. Dermatol. 67, 658–664 (2012).
Schwartz, D. M. et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 16, 843–862 (2017).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dainichi, T., Kitoh, A., Otsuka, A. et al. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat Immunol 19, 1286–1298 (2018). https://doi.org/10.1038/s41590-018-0256-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-018-0256-2
This article is cited by
-
Disease modification in inflammatory skin disorders: opportunities and challenges
Nature Reviews Drug Discovery (2023)
-
Natural alternatives targeting psoriasis pathology and key signaling pathways: a focus on phytochemicals
Phytochemistry Reviews (2023)
-
Emerging trends in combination strategies with phototherapy in advanced psoriasis management
Inflammopharmacology (2023)
-
A multifunctional composite hydrogel as an intrinsic and extrinsic coregulator for enhanced therapeutic efficacy for psoriasis
Journal of Nanobiotechnology (2022)
-
Mupirocin blocks imiquimod-induced psoriasis-like skin lesion by inhibiting epidermal isoleucyl-tRNA synthetase
Cell Communication and Signaling (2022)