Abstract
Nuclear pore complexes (NPCs) are channels connecting the nucleus with the cytoplasm. We report that loss of the tissue-specific NPC component Nup210 causes a severe deficit of naïve CD4+ T cells. Nup210-deficient CD4+ T lymphocytes develop normally but fail to survive in the periphery. The decreased survival results from both an impaired ability to transmit tonic T cell receptor (TCR) signals and increased levels of Fas, which sensitize Nup210–/– naïve CD4+ T cells to Fas-mediated cell death. Mechanistically, Nup210 regulates these processes by modulating the expression of Cav2 (encoding Caveolin-2) and Jun at the nuclear periphery. Whereas the TCR-dependent and CD4+ T cell–specific upregulation of Cav2 is critical for proximal TCR signaling, cJun expression is required for STAT3-dependent repression of Fas. Our results uncover an unexpected role for Nup210 as a cell-intrinsic regulator of TCR signaling and T cell homeostasis and expose NPCs as key players in the adaptive immune system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Takada, K. & Jameson, S. C. Naive T cell homeostasis: from awareness of space to a sense of place. Nat. Rev. Immunol. 9, 823–832 (2009).
Surh, C. D. & Sprent, J. Homeostasis of naive and memory T cells. Immunity 29, 848–862 (2008).
Viret, C., Wong, F. S. & Janeway, C. A. Jr. Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity 10, 559–568 (1999).
Blattman, J. N. et al. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J. Exp. Med. 195, 657–664 (2002).
Beck, M. & Hurt, E. The nuclear pore complex: understanding its function through structural insight. Nat. Rev. Mol. Cell Biol. 18, 73–89 (2017).
Ibarra, A. & Hetzer, M. W. Nuclear pore proteins and the control of genome functions. Genes Dev. 29, 337–349 (2015).
Raices, M. & D’Angelo, M. A. Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions. Nat. Rev. Mol. Cell Biol. 13, 687–699 (2012).
Olsson, M., Schéele, S. & Ekblom, P. Limited expression of nuclear pore membrane glycoprotein 210 in cell lines and tissues suggests cell-type specific nuclear pores in metazoans. Exp. Cell Res. 292, 359–370 (2004).
D’Angelo, M. A., Gomez-Cavazos, J. S., Mei, A., Lackner, D. H. & Hetzer, M. W. A change in nuclear pore complex composition regulates cell differentiation. Dev. Cell 22, 446–458 (2012).
Raices, M. et al. Nuclear pores regulate muscle development and maintenance by assembling a localized Mef2C complex. Dev. Cell 41, 540–554.e547 (2017).
Pascual-Garcia, P. et al. Metazoan nuclear pores provide a scaffold for poised genes and mediate induced enhancer-promoter contacts. Mol. Cell 66, 63–76.e66 (2017).
D’Urso, A. & Brickner, J. H. Epigenetic transcriptional memory. Curr. Genet. 63, 435–439 (2017).
Heng, T. S. & Painter, M. W. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094 (2008).
Yu, J. et al. Regulation of T-cell activation and migration by the kinase TBK1 during neuroinflammation. Nat. Commun. 6, 6074 (2015).
Sebzda, E., Zou, Z., Lee, J. S., Wang, T. & Kahn, M. L. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nat. Immunol. 9, 292–300 (2008).
Aghajani, K., Keerthivasan, S., Yu, Y. & Gounari, F. Generation of CD4CreER(T2) transgenic mice to study development of peripheral CD4-T-cells. Genesis 50, 908–913 (2012).
Bradley, L. M., Watson, S. R. & Swain, S. L. Entry of naive CD4 T cells into peripheral lymph nodes requires L-selectin. J. Exp. Med. 180, 2401–2406 (1994).
Carrette, F. & Surh, C. D. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin. Immunol. 24, 209–217 (2012).
Labrecque, N. et al. How much TCR does a T cell need? Immunity 15, 71–82 (2001).
Martin, B., Bécourt, C., Bienvenu, B. & Lucas, B. Self-recognition is crucial for maintaining the peripheral CD4+ T-cell pool in a nonlymphopenic environment. Blood 108, 270–277 (2006).
Brocker, T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expressing dendritic cells. J. Exp. Med. 186, 1223–1232 (1997).
Seddon, B. & Zamoyska, R. TCR signals mediated by Src family kinases are essential for the survival of naive T cells. J. Immunol. 169, 2997–3005 (2002).
Macian, F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 5, 472–484 (2005).
Foletta, V. C., Segal, D. H. & Cohen, D. R. Transcriptional regulation in the immune system: all roads lead to AP-1. J. Leukoc. Biol. 63, 139–152 (1998).
Brownlie, R. J. & Zamoyska, R. T cell receptor signalling networks: branched, diversified and bounded. Nat. Rev. Immunol. 13, 257–269 (2013).
Nika, K. et al. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity 32, 766–777 (2010).
Cho, J. H. et al. CD45-mediated control of TCR tuning in naïve and memory CD8+T cells. Nat. Commun. 7, 13373 (2016).
Liu, Z. G., Smith, S. W., McLaughlin, K. A., Schwartz, L. M. & Osborne, B. A. Apoptotic signals delivered through the T-cell receptor of a T-cell hybrid require the immediate-early gene nur77. Nature 367, 281–284 (1994).
Au-Yeung, B. B. et al. A sharp T-cell antigen receptor signaling threshold for T-cell proliferation. Proc. Natl. Acad. Sci. USA 111, E3679–E3688 (2014).
Moran, A. E. et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208, 1279–1289 (2011).
Bunnell, S. C. et al. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J. Cell Biol. 158, 1263–1275 (2002).
Fooksman, D. R. et al. Functional anatomy of T cell activation and synapse formation. Annu. Rev. Immunol. 28, 79–105 (2010).
Schönle, A. et al. Caveolin-1 regulates TCR signal strength and regulatory T-cell differentiation into alloreactive T cells. Blood 127, 1930–1939 (2016).
Song, K. S. et al. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells: caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J. Biol. Chem. 271, 15160–15165 (1996).
Scherer, P. E. et al. Cell-type and tissue-specific expression of caveolin-2: caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J. Biol. Chem. 272, 29337–29346 (1997).
Tomassian, T. et al. Caveolin-1 orchestrates TCR synaptic polarity, signal specificity, and function in CD8 T cells. J. Immunol. 187, 2993–3002 (2011).
Sandberg, Y. et al. Human T-cell lines with well-defined T-cell receptor gene rearrangements as controls for the BIOMED-2 multiplex polymerase chain reaction tubes. Leukemia 21, 230–237 (2007).
Li, Q. et al. Critical role of histone demethylase Jmjd3 in the regulation of CD4+ T-cell differentiation. Nat. Commun. 5, 5780 (2014).
Wei, G. et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167 (2009).
Bernstein, B. E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006).
Ivanov, V. N. et al. Cooperation between STAT3 and c-jun suppresses Fas transcription. Mol. Cell 7, 517–528 (2001).
Liu, H. et al. The immune adaptor SLP-76 binds to SUMO-RANGAP1 at nuclear pore complex filaments to regulate nuclear import of transcription factors in T cells. Mol. Cell 59, 840–849 (2015).
Faria, A. M. et al. The nucleoporin Nup96 is required for proper expression of interferon-regulated proteins and functions. Immunity 24, 295–304 (2006).
Moreira, T. G. et al. Sec13 regulates expression of specific immune factors involved in inflammation in vivo. Sci. Rep. 5, 17655 (2015).
Gu, Y. et al. Nuclear pore permeabilization is a convergent signaling event in effector-triggered immunity. Cell 166, 1526–1538.e1511 (2016).
Ohnuma, K. et al. Caveolin-1 triggers T-cell activation via CD26 in association with CARMA1. J. Biol. Chem. 282, 10117–10131 (2007).
Gabriel, C. H. et al. Identification of novel nuclear factor of activated T cell (NFAT)-associated proteins in T cells. J. Biol. Chem. 291, 24172–24187 (2016).
Greber, U. F., Senior, A. & Gerace, L. A major glycoprotein of the nuclear pore complex is a membrane-spanning polypeptide with a large lumenal domain and a small cytoplasmic tail. EMBO J. 9, 1495–1502 (1990).
Yewdell, W. T., Colombi, P., Makhnevych, T. & Lusk, C. P. Lumenal interactions in nuclear pore complex assembly and stability. Mol. Biol. Cell 22, 1375–1388 (2011).
Lapetina, D. L., Ptak, C., Roesner, U. K. & Wozniak, R. W. Yeast silencing factor Sir4 and a subset of nucleoporins form a complex distinct from nuclear pore complexes. J. Cell Biol. 216, 3145–3159 (2017).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014).
Kerdiles, Y. M. et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat. Immunol. 10, 176–184 (2009).
Severgnini, M. et al. A rapid two-step method for isolation of functional primary mouse hepatocytes: cell characterization and asialoglycoprotein receptor based assay development. Cytotechnology 64, 187–195 (2012).
Mei, S. et al. Cistrome Data Browser: a data portal for ChIP-Seq and chromatin accessibility data in human and mouse. Nucleic Acids Res. 45, D658–D662 (2017).
Kent, W. J. et al. The human genome browser at UCSC. Genome Res. 12, 996–1006 (2002).
Acknowledgements
We thank L. E. Samelson (National Institutes of Health) for kindly providing the SLP-76-EYFP cell line and M. Hetzer (Salk Institute) for kindly providing the anti-Nup107 antibody. J.B. was supported by American Heart Association Award 15POST22600000 and an SBP Fishman Fund Fellowship. M.A.D. was supported by a Pew Biomedical Science Scholar Award and Research Scholar Grant RSG-17-148-01-CCG from the American Cancer Society. This work was also supported by the National Institutes of Health (awards RO1AR065083 and RO1AR065083-S1). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was additionally supported by the NCI Cancer Center grant P30 CA030199, which supports the animal, flow cytometry, genomics, and bioinformatics cores at the SBP La Jolla campus. The electron microscopy work was supported by the Waitt Advanced Biophotonics Core Facility of the Salk Institute with funding from NIH-NCI CCSG: P30 014195, an NINDS Neuroscience Core Grant and the Waitt Foundation.
Author information
Authors and Affiliations
Contributions
J.B. designed the experimental approach, performed experiments, analyzed data, and cowrote the manuscript; S.S. performed experiments, analyzed data, and provided critical input; M.R. performed experiments, analyzed data, and provided critical input; F.C. and R.T. assisted with adoptive transfer experiments and provided critical input; L.M.B. provided reagents and critical expertise; M.A.D. designed the experimental approach, analyzed data, provided oversight and critical expertise, and cowrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Nup210–/– cells have functional NPCs.
(a) Nup210 mRNA levels in wild type Fluorescently Activated Cell Sorted mouse splenic B, CD4+ and CD8+ T lymphocytes, eosinophils (Eos), and bone marrow macrophages (Mac), Monocytes (Mono) and Neutrophils (Neut) were determined by qPCR. Nup210 expression was normalized to that of Hprt and Gapdh. (b) Schematic diagram depicting the strategy used to generate Nup210f/f mice (E: exon). LoxP sites flanking exon 2 of Nup210 are indicated. Start and stop codons are indicated above, and chromosomal locations are shown below. (c) Western blot analysis of Nup210 protein levels in Nup210+/+ and Nup210–/– mouse livers using an antibody targeting the C-terminal domain of Nup210. Hsp90 was used as loading control. Molecular mass markers are shown on the left. (d) Nuclei isolated from Nup210+/+ or Nup210–/– primary hepatocytes were incubated with different NPC antibodies and co-stained with Hoechst. (e,f) Primary hepatocyte cells derived from Nup210+/+ and Nup210–/– mice were transfected with the NES-Tomato-NLS reporter and nuclear import was analyzed by FRAP, as described9. (e) Schematic illustration of FRAP experiments. (f) Representative images of NES-Tomato-NLS-transfected hepatocytes before and after photobleaching. Data are representative of (a) one, (c,d,f) two independent experiments. (a) n = 3 technical replicates of one biological sample prepared pooling cells from n = 3 mice.
Supplementary Figure 2 Nup210 depletion reduces naïve CD4+ T lymphocyte number.
(a) Percentage of neutrophils (Ly6G+Ly6CloCD11b+), monocytes (Ly6G-Ly6ChiCD11b+) and eosinophils (Siglec-F+CD11b+SSChi) in bone marrow of Nup210+/+ and Nup210–/– mice. (b) Splenic B lymphocyte (B220+CD3-) numbers. (c) Total splenocyte numbers. (d–f) The percentage of CD3+ T cells (d), CD4+/CD8+ T cell ratios (e) and the percentage of naïve (CD44lo) and memory (CD44hi) CD4+ T cells (f) were analyzed in blood of Nup210+/+ and Nup210–/– mice. (g–j) Flow cytometry analysis of Nup210+/+ and Nup210–/– spleens. (g) Number of splenic CD3+TCRγδ T cells. (h) Number of splenic NK (NK1.1+CD49b+CD3-) cells. (i) Number of splenic NKT (NK1.1+CD3+) cells. (j) Number of splenic regulatory T (CD4+Foxp3+) cells. (a–j) mean ± s.e.m. Each symbol (a–j) represents an individual mouse. Data are representative of (a) four, (d–f,h,i) one, and (g) two independent experiments, or are pooled from (b,j) two, and (c) twelve independent experiments. Mice per group (a) n = 3 or 4, (b) n = 8, (c) n = 41 or 46, (d–f) n = 4 or 5, (g-i) n = 4 mice, (j) n = 7 or 8. NS, not significant (P > 0.05); *P ≤ 0.05, ***P ≤ 0.001 (two-tailed unpaired Student’s t-test).
Supplementary Figure 3 Nup210–/– mice have normal T cell development and peripheral naïve CD4+ T cell circulation.
(a–d) Flow cytometry analysis of thymic populations in Nup210+/+ and Nup210–/– mice. (a) Number of total thymocytes. (b) Percentage of double-positive (DP), double-negative (DN), CD4+ and CD8+ single-positive thymocytes. (c) CD4+ and CD8+ thymocyte numbers. (d) Number of thymic naïve (CD44lo) and memory (CD44hi) CD4+ T cells. (e–g) Flow cytometry analysis of mesenteric lymph nodes (mLN) of Nup210+/+ and Nup210–/– mice. (e) Number of CD4+ and CD8+ T cells. (f) CD4+/CD8+ T cell ratios. (g) Naïve/memory CD4+ T cell numbers. (a–g) mean ± s.e.m. Each symbol (a–g) represents an individual mouse. Data are pooled from (a–c,e,f) four, (d,g) three independent experiments. Mice per group (a–c) n = 14 or 15, (d) n = 11, (e) n = 10 or 13, (f) n = 12 or 14, (g) n = 8 or 11. NS, not significant (P > 0.05); ***P ≤ 0.001, ****P ≤ 0.0001 (two-tailed unpaired Student’s t-test).
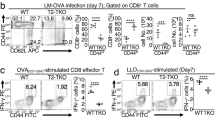
Supplementary Figure 4 Nup210–/– naïve CD4+ T cells have reduced survival.
(a) CFSE-labeled Nup210+/+ and CTV-labeled Nup210–/– T cells were co-transferred into lymphoreplete Nup210+/+ hosts. CD4+CD44lo T cell ratio at time of transfer (Transf) and 7 days later in spleens (Spl), peripheral (pLN) and mesenteric lymph nodes (mLN) is shown. (b) Percentage of dead naïve CD4+ (CD4+CD44lo) T cells in mLN. (a,b) mean ± s.e.m. Each symbol (a,b) represents an individual mouse. Data are representative of (a) two independent experiments or are pooled from (b) three independent experiments. (a) n = 4 host mice, (b) n = 9 mice per group. *P ≤ 0.05 (two-tailed unpaired Student’s t-test).
Supplementary Figure 5 Nup210 is dispensable for naïve CD4+ T cell homing.
(a) CCR7 and CD62L mean fluorescence intensity (MFI) in CD4+CD44lo T cells are represented as a percentage of Nup210+/+ cells. (b) CFSE-labeled Nup210+/+ and CTV-labeled Nup210–/– T cells were co-transferred into lymphoreplete Nup210+/+ hosts. CD4+CD44lo T cell ratio at time of transfer (Transf) and 18 hours later in peripheral (pLN) and mesenteric (mLN) lymph nodes is shown. (c) Sorting strategy used to FACS-purify naïve CD4+ (CD4+CD62LhiCD44loCD25-) T cells from spleens and pLN (cervical, axillary, brachial and inguinal) of mice. Representative pre- and post-sort samples are shown. Frequencies for each population are indicated. (a,b) mean ± s.e.m. Each symbol represents an individual mouse. Data are pooled from (a) three independent experiments or are representative of (b) two independent experiments. (a) n = 9 mice per group, (b) n = 3 or 4 host mice. NS, not significant (P > 0.05); ***P ≤ 0.001, (two-tailed unpaired Student’s t-test).
Supplementary Figure 6 TCR signaling is compromised in Nup210–/– naïve CD4+ T cells.
(a) Flow cytometry analysis of Zap70 (pY319)/Syk (pY352) expression in resting naïve CD4+ (CD4+CD62LhiCD44loCD25-) T cells. Sodium peroxyvanadate activation was used as positive control. Secondary Ab: fluorescently labeled secondary antibody stained sample. (b) Mean Fluorescence Intensity (MFI) values from (a) as a percentage of Nup210+/+ levels. (c) Gating strategy used to define CD69+ cells after TCR stimulation with anti-CD3, anti-CD28, and IL-2. Frequencies for each population are shown. Representative samples are shown. (d) Percentage of CD4+CD69+ T cells after TCR activation with plate-bound anti-CD3 and anti-CD28 antibodies in the presence of IL-2. (b,d) mean ± s.e.m. Each symbol represents an individual mouse. Data are representative of (a,b) three, (c) two independent experiments, or are pooled from (d) two independent experiments. Mice per group: (b) n = 13 or 14, (d) n = 5, 6 or 7. NS, not significant (P > 0.05); *P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001 (two-tailed unpaired Student’s t-test).
Supplementary Figure 7 Cav2 overexpression rescues proximal TCR signaling in NUP210–/– T cells.
(a) qPCR analysis of cFOS, CD69, NFATC1, NUR77, and EGR2 mRNA levels in unstimulated or soluble anti-CD3 activated NUP210+/+ or NUP210–/– J14 SLP-76-EYFP cells. Expression was normalized to that of HPRT1. (b) NUP210+/+ or NUP210–/– J14 SLP-76-EYFP cells were transduced with lentiviruses expressing control (empty vector) or Cav2, and either left unstimulated or activated with soluble anti-CD3, followed by staining with anti-Cav2 antibody. Representative images of maximal projections of SLP-76 and Cav2 immunofluorescence are shown. (c) qPCR analysis of CD69, cFOS, NUR77, EGR2, IL2, and NFATC1 mRNA levels in unstimulated or soluble anti-CD3-activated NUP210+/+ or NUP210–/– J14 SLP-76-EYFP cells transduced with lentiviruses expressing control (Ctr) or Cav2 vectors. Expression was normalized to that of HPRT1. (a,c) mean ± s.d. Each symbol represents a technical replicate. Data are representative of (a) three, (b) four, (c) two independent experiments.
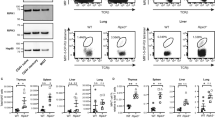
Supplementary Figure 8 Cav2 expression is induced with TCR stimulation, and Nup210 depletion causes increased sensitivity to Fas-mediated apoptosis.
(a) Scheme of the quantification of DNA-FISH experiments. (b) H3K4me3 and H3K27me3 marks on the Cav2 and Cav1 genes in mouse naïve CD4+ T cells were analyzed using the UCSC genome browser and data from Li, Q. et al.38 and Wei, G. et al.39. Boxes highlight H3K4me3 (red) or H3K27me3 (green) peaks on the gene promoters. (c) Cav2 mRNA levels in wild type mouse naïve CD4+ or CD8+ T cells unstimulated or activated with soluble anti-CD3 were determined by qPCR. Expression was normalized to that of Hprt. (d) Western blot analysis of STAT3 protein levels in NUP210+/+ or NUP210–/– J14 SLP-76-EYFP cells. Hsp90 was used as loading control. (e) Unstimulated NUP210+/+ or NUP210–/– J14 SLP-76-EYFP cells were incubated with the indicated antibodies and co-stained with Hoechst. (f) NUP210+/+ or NUP210–/– J14 SLP-76-EYFP cells were incubated for 16-24 hours with the indicated concentrations of soluble Fas ligand (FasL). The percentage of cells stained for both annexin V and propidium iodide (late apoptosis/necrosis) is shown. (g) Model for Nup210 function. In CD4+ T cells, Nup210 is required for the expression of Cav2 and Jun genes at the nuclear periphery. Cav2 expression is induced by TCR activation and required for proximal TCR signaling and the transmission of survival and activation signals. cJun expression is required for the repression of Fas with its partner STAT3. In the absence of Nup210, early TCR signaling is impaired, preventing the transmission of survival signals; and STAT3 recruitment to the Fas promoter is reduced, resulting in higher levels of Fas at the plasma membrane and increased sensitivity to Fas-mediated apoptosis. (c) mean ± s.e.m; (f) mean ± s.d.; each symbol represents (c) an individual mouse or (f) a technical replicate. Data are representative of (c,f) one, (d) three, (e) two independent experiments. (c) n = 3 mice per group, (f) n = 2 technical replicates of one biological sample from each group. NS, not significant (P > 0.05); **P ≤ 0.01 (two-tailed unpaired Student’s t-test).
Supplementary information
Supplementary Figures
Supplementary Figures 1–8
Supplementary Table 1: List of the differentially expressed genes in Nup210+/+ and Nup210–/– naïve CD4+ T cells
Data were collected from three independent experiments. A total of 3 biological replicates from each genotype, each prepared pooling cells from n = 4 mice per group, were sequenced. Differential transcript expression was determined using the Cufflinks Cuffdiff package (http://cufflinks.cbcb.umd.edu/). False discovery rate (FDR) adjusted P values (q values) were calculated using the Benjamini-Hochberg correction for multiple testing with an allowed false discovery rate of 0.05. Further details can be found in the Methods section.
Rights and permissions
About this article
Cite this article
Borlido, J., Sakuma, S., Raices, M. et al. Nuclear pore complex-mediated modulation of TCR signaling is required for naïve CD4+ T cell homeostasis. Nat Immunol 19, 594–605 (2018). https://doi.org/10.1038/s41590-018-0103-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-018-0103-5
This article is cited by
-
Nuclear pore protein NUP210 depletion suppresses metastasis through heterochromatin-mediated disruption of tumor cell mechanical response
Nature Communications (2021)
-
Disease-specific alteration of karyopherin-α subtype establishes feed-forward oncogenic signaling in head and neck squamous cell carcinoma
Oncogene (2020)