Abstract
Although deletion of certain autophagy-related genes has been associated with defects in hematopoiesis, it remains unclear whether hyperactivated mitophagy affects the maintenance and differentiation of hematopoietic stem cells (HSCs) and committed progenitor cells. Here we report that targeted deletion of the gene encoding the AAA+-ATPase Atad3a hyperactivated mitophagy in mouse hematopoietic cells. Affected mice showed reduced survival, severely decreased bone-marrow cellularity, erythroid anemia and B cell lymphopenia. Those phenotypes were associated with skewed differentiation of stem and progenitor cells and an enlarged HSC pool. Mechanistically, Atad3a interacted with the mitochondrial channel components Tom40 and Tim23 and served as a bridging factor to facilitate appropriate transportation and processing of the mitophagy protein Pink1. Loss of Atad3a caused accumulation of Pink1 and activated mitophagy. Notably, deletion of Pink1 in Atad3a-deficient mice significantly ‘rescued’ the mitophagy defect, which resulted in restoration of the progenitor and HSC pools. Our data indicate that Atad3a suppresses Pink1-dependent mitophagy and thereby serves a key role in hematopoietic homeostasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Wallace, D. C. & Fan, W. Energetics, epigenetics, mitochondrial genetics. Mitochondrion 10, 12–31 (2010).
Sun, M. G. et al. Correlated three-dimensional light and electron microscopy reveals transformation of mitochondria during apoptosis. Nat. Cell Biol. 9, 1057–1065 (2007).
Salminen, A., Kaarniranta, K., Hiltunen, M. & Kauppinen, A. Krebs cycle dysfunction shapes epigenetic landscape of chromatin: novel insights into mitochondrial regulation of aging process. Cell. Signal. 26, 1598–1603 (2014).
Kim, H. R. et al. Mitochondrial DNA aberrations of bone marrow cells from patients with aplastic anemia. J. Korean Med. Sci. 23, 1062–1067 (2008).
Kim, H. R. et al. Mitochondrial DNA aberrations and pathophysiological implications in hematopoietic diseases, chronic inflammatory diseases, and cancers. Ann. Lab. Med. 35, 1–14 (2015).
Yao, Y. G. et al. Mitochondrial DNA sequence variation in single cells from leukemia patients. Blood 109, 756–762 (2007).
Joshi, A. & Kundu, M. Mitophagy in hematopoietic stem cells: the case for exploration. Autophagy 9, 1737–1749 (2013).
Mantel, C., Messina-Graham, S. & Broxmeyer, H. E. Upregulation of nascent mitochondrial biogenesis in mouse hematopoietic stem cells parallels upregulation of CD34 and loss of pluripotency: a potential strategy for reducing oxidative risk in stem cells. Cell Cycle 9, 2008–2017 (2010).
Guan, J. L. et al. Autophagy in stem cells. Autophagy 9, 830–849 (2013).
Gan, B. et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature 468, 701–704 (2010).
Nakada, D., Saunders, T. L. & Morrison, S. J. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature 468, 653–658 (2010).
Gurumurthy, S. et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature 468, 659–663 (2010).
Liu, F. et al. FIP200 is required for the cell-autonomous maintenance of fetal hematopoietic stem cells. Blood 116, 4806–4814 (2010).
Mortensen, M. et al. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J. Exp. Med. 208, 455–467 (2011).
Ho, T. T. et al. Autophagy maintains the metabolism and function of young and old stem cells. Nature 543, 205–210 (2017).
Ito, K. et al. Self-renewal of a purified Tie2+ hematopoietic stem cell population relies on mitochondrial clearance. Science 354, 1156–1160 (2016).
Wei, H., Liu, L. & Chen, Q. Selective removal of mitochondria via mitophagy: distinct pathways for different mitochondrial stresses. Biochim. Biophys. Acta 1853, 2784–2790 (2015).
Novak, I. et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 11, 45–51 (2010).
Liu, L. et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 14, 177–185 (2012).
Narendra, D. P. et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8, e1000298 (2010).
Matsuda, N. et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 189, 211–221 (2010).
Greene, A. W. et al. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep. 13, 378–385 (2012).
Pickrell, A. M. & Youle, R. J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85, 257–273 (2015).
Gilquin, B. et al. The AAA + ATPase ATAD3A controls mitochondrial dynamics at the interface of the inner and outer membranes. Mol. Cell. Biol. 30, 1984–1996 (2010).
Goller, T., Seibold, U. K., Kremmer, E., Voos, W. & Kolanus, W. Atad3 function is essential for early post-implantation development in the mouse. PLoS One 8, e54799 (2013).
Harel, T. et al. Recurrent de novo and biallelic variation of ATAD3A, encoding a mitochondrial membrane protein, results in distinct neurological syndromes. Am. J. Hum. Genet. 99, 831–845 (2016).
Kühn, R., Schwenk, F., Aguet, M. & Rajewsky, K. Inducible gene targeting in mice. Science 269, 1427–1429 (1995).
Kiel, M. J. et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005).
Oguro, H., Ding, L. & Morrison, S. J. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell 13, 102–116 (2013).
Foudi, A. et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat. Biotechnol. 27, 84–90 (2009).
Challen, G. A., Boles, N., Lin, K. K. & Goodell, M. A. Mouse hematopoietic stem cell identification and analysis. Cytometry A 75, 14–24 (2009).
Miyamoto, K. et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell 1, 101–112 (2007).
Tothova, Z. et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325–339 (2007).
Lelliott, C. J. & Vidal-Puig, A. PGC-1beta: a co-activator that sets the tone for both basal and stress-stimulated mitochondrial activity. Adv. Exp. Med. Biol. 646, 133–139 (2009).
Austin, S. & St-Pierre, J. PGC1α and mitochondrial metabolism-emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 125, 4963–4971 (2012).
Tal, M. C. et al. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc. Natl. Acad. Sci. USA 106, 2770–2775 (2009).
Narendra, D., Tanaka, A., Suen, D. F. & Youle, R. J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803 (2008).
Jin, S. M. et al. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. 191, 933–942 (2010).
Lazarou, M. et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524, 309–314 (2015).
Vannini, N. et al. Specification of haematopoietic stem cell fate via modulation of mitochondrial activity. Nat. Commun. 7, 13125 (2016).
Mortensen, M. et al. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc. Natl. Acad. Sci. USA 107, 832–837 (2010).
Sandoval, H. et al. Essential role for Nix in autophagic maturation of erythroid cells. Nature 454, 232–235 (2008).
Skarnes, W. C. et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature 474, 337–342 (2011).
Hubstenberger, A., Labourdette, G., Baudier, J. & Rousseau, D. ATAD 3 A and ATAD 3B are distal 1p-located genes differentially expressed in human glioma cell lines and present in vitro anti-oncogenic and chemoresistant properties. Exp. Cell Res. 314, 2870–2883 (2008).
Wang, X. et al. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 147, 893–906 (2011).
Acknowledgements
We thank X. Lin (MD Anderson Cancer Center) for the for FLP transgenic mouse; T.L. Schwarz (Boston Children’s Hospital) for the Pink1-encoding plasmid; J. Baudier (Université d1Aix-Marseille) and D. Rousseau (University Grenoble Alpes) for antibodies to Atad3a; J. Parker-Thornburg and J. Alana for blastocyst microinjection; D.P. Pollock for microarray analysis; K. Dunner for transmission-electron-microscopy imaging; B. Zhen and S. Zhang for technical assistance; the flow cytometry core facility at MD Anderson Cancer Center and Wake Forest School of Medicine for flow cytometry analysis; D.D. Sarbassov and the members of the Lin laboratory for comments and suggestions; and X. Zhou for the assistance in statistical analysis. Supported by the National Key Research and Development Program of China (2016YFA0101200 to X.-W.B.), the National Natural Science Foundation of China (61327902-04 to X.-W.B.), the US National Institutes of Health (R01 CA182424 and R01 CA193813 to H.-K.L.) and Wake Forest University School of Medicine (start-up funds to H.-K.L.).
Author information
Authors and Affiliations
Contributions
G.J., C.X., X.-Z. and J.L. performed the experiments; A.H.R., C.L., S.K. and B.P. provided technical support, critical comments and suggestions; M.E.F. edited the paper; and G.J., X.-W.B. and H.-K.L. designed the experiments, analyzed the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
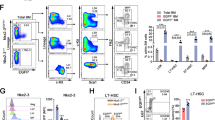
Supplementary Figure 1 Atad3a regulates bone-marrow hematopoiesis
(a) KOMP Atad3a targeting ES cells are ordered to generate Atad3a knockout-first mice, which are then bred with Flp mice to generate Atad3a condition knockout mice Atad3a fl/fl, and further bred with Mx1Cre mice to establish Atad3a fl/flMx1Cre mice. Atad3a fl/flMx1Cre mice are treated with Poly(I:C) to induce Cre activity and disrupt Atad3a, thus knockout Atad3a in bone marrow hematopoietic cells. (b) Immunoblotting analysis of indicated proteins in total bone marrow (BM) and sorted lineage negative (LinNeg) cells isolated from Poly(I:C) treated control (Atad3a fl/fl) and Atad3a deficient (Atad3a fl/flMx1Cre) mice. Western blot data are representative results from two independent experiments. (c) The percentages of bone marrow lineage cells. Values are means ± SD. n = 3 mice. Student’s t-test. ns indicates no significance. (d) Representative flow cytometry plots of Ter119+ erythroids, B220+ lymphocytes and Gr1+Mac1+ myeloids in bone marrow. Numbers in the plots indicates the percentages of gated cells.
Supplementary Figure 2 Bone-marrow-transplantation assay
(a) The percentages of donor derived CD45.2+ peripheral blood cells 5 weeks after non-competitive transplantation (n > = 4 mice). Student’s t-test. ns indicates non-significant. (b) Representative flow cytometry plots of CD45.2+ bone marrow cells and erythroid, B, myeloid cells in non-competitive transplantation bone marrow. (c) Representative flow cytometry plots of donor derived (CD45.2+) B220+ and Mac1+ cells in competitive transplantation bone marrow. (d) Ter119+ cells are negative for both CD45.1 and CD45.2 in competitive bone marrow transplanted mice. Numbers in the plots indicate the percentages of gated cells (b-d). (e) The percentage of CD45.2+ cells in B220+ blood cells. (f) The percentage of CD45.2+ cells in Mac1+ blood cells. (g) The percentages of CD45.2+ cells in bone marrow B220+ or Mac1+ cells. Values in (e-g) are means ± SD. n > = 4 mice. Student’s t-test. *p < 0.05; **p < 0.01; ***p < 0.001.
Supplementary Figure 3 Deletion of Atad3a leads to hematopoietic defects
(a) Gating strategy of CD48–CD150+ cells in total bone marrow. (b) The percentages of HSC and MPP in LSK compartment. Values are means ± SD (n = 8 mice). The results are averaged from two independent experiments. Student’s t-test. ***p < 0.001. (c) Proliferation assay by measuring BrdU incorporation in the primary cultured lineage negative bone marrow cells. Values are means ± SD (n = 3). Representative results are shown from two independent experiments. Student’s t-test. *p < 0.05. (d) TUNEL apoptosis assay of sorted lineage negative cells. Values are means ± SD (n = 3). Representative results are shown from two independent experiments. Student’s t-test. ***p < 0.001. (e) The percentages of CMP in LS-K compartment. Values are means ± SD (n = 6 mice). The results are averaged from two independent experiments. Student’s t-test. ***p < 0.001. (f) Representative flow cytometry plots of CLPs. LSlowKlow indicates lineage negative Sca-1lowcKitlow cells. (g) Representative flow cytometry plots of erythroid cells at different differentiation stages, including Ter119medCD71hi proerythroblasts (I), Ter119hiCD71hi basophilic erythroblasts (II) and Ter119hiCD71med/low late erythroblasts (III&IV). (h) CFU formation assay of CMPs and MEPs. Values are means ± SD. n = 5. Student’s t-test. ***p < 0.001. (i) Representative flow cytometry plots of B220+CD19–CD24–CD43+IgM– pre-pro B, B220+CD19+CD24+CD43+IgM– pro B, B220+CD19+CD24+CD43–IgM– pre B and B220+CD19+CD24+CD43–IgM+ immature B cells. Numbers in the plots indicate the percentages of gated cells (f, g, i).
Supplementary Figure 4 The role of Atad3a in mitochondrial regulation
(a) Representative flow cytometry plots of Mitotracker green GM staining. (b) Cellular ROS production in hematopoietic cell populations. LinNeg indicates lineage negative cells. (c) Cellular ATP concentration in sorted lineage negative cells. (d) RT-PCR analysis of transcription factors and co-activators involved in mitochondrial biogenesis (left) and nuclear encoded mitochondrial proteins (right) in sorted lineage negative cells. Statistical values shown in (b-d) are means ± SD (n = 3). Representative results are shown from at least two independent experiments. Student’s t-test. ns in indicates non-significant. (e) Quantification of mitophagy-dependent mitochondrial mass reduction in Atad3a deficient hematopoietic cell populations in the absence and presence of Bafilomycin A1 (Bafi). LinNeg indicates lineage negative cells. Values are means ± SD (n = 3 mice). Representative results are shown from two independent experiments. One-way ANOVA followed by Bonferroni post test. *p < 0.05; **p < 0.01; ***p < 0.001.
Supplementary Figure 5 Atad3a facilitates the import of Pink1 into mitochondria
(a) The integrity of purified mitochondria was confirmed by Cytochrome C (CytC) retaining in the mitochondrial fraction but not in the cytosol. (b) Immunoblotting analysis of indicated proteins in the total lysate of sorted lineage negative cells. Ratio indicates the Pink1/β-actin ratio. (c) Mitochondrial mass analysis in control and Pink1 knockdown HEK293T cells. Values are means ± SD (n = 3). Representative results are shown from two independent experiments. Student’s t-test. ns indicates non-significant. (d) Co-immunoprecipitation analysis of the interaction between Atad3a and Pink1 in HEK293T cells. (e) In vitro binding assay between purified Flag-Pink1 and myc-Atad3a. (f) Schematic model to demonstrate that loss of mitochondrial membrane potential disrupts the interaction of Atad3a with TIM complex, therefore preventing Pink1 transportation from TOM complex to TIM complex and accumulating Pink1 on the outer membrane. (g) Co-immunoprecipitation analysis of the interaction between Atad3a and Pink1 with or without CCCP treatment in HEK293T cells. CCC treatment: 20μM. The Western blot data are representative results from two (a, e, g) or three (b, d) independent experiments. (h) Schematic model of Atad3a-mediated Pink1 importation and mitophagy suppression. Atad3a serves as a key bridging factor between TOM complex and TIM complex to facilitate Pink1 transportation from outer membrane to inner membrane in wild-type cell mitochondria. Once imported, Pink1 is sequentially cleaved by MPP and PARL peptidases and then subjected to final degradation. Loss of Atad3a in Atad3a knockout cells blocks the Pink1 mitochondrial importation and accumulates full-length Pink1 on the mitochondrial OMM, therefore triggering Pink1-mediated mitophagy. OMM: outer mitochondrial membrane; IMM; inner mitochondrial membrane.
Supplementary Figure 6 Atad3a-mediated of mitophagy is critical for stem-cell and progenitor-cell homeostasis
(a) Representative flow cytometry plots of CLPs in mouse bone marrow. (b) Representative flow cytometry plots of LT-HSCs, ST-HSCs, MPPs and LSKs in mouse bone marrow. (c) Representative flow cytometry plots of CMPs, MEPs and GMPs in mouse bone marrow. (d) Representative flow cytometry plots of CLPs in mouse bone marrow. LSlowKlow indicates lineage negative Sca-1lowcKitlow cells. Numbers in the plots indicates the percentages of gated cells.
Supplementary Figure 7 The role of Atad3a-mediated suppression of Pink1-dependent mitophagy in lineage cells
(a) Representative flow cytometry plots of the lineage cells in mouse bone marrow. Numbers in the plots indicate the percentages of gated cells. (b-e) The percentages (b, d) or cell numbers (c, e) of lineage cells in mouse bone marrow. CLQ indicates Chloroquine; Values are means ± SD (n = 3-5 mice). Representative results are shown from two independent experiments with biological replicates. One-way ANOVA followed by Bonferroni post test. ***p < 0.001.
Supplementary Figure 8
Full-length scans of immunoblots in the main figures
Supplementary information
Integrated Supplementary Figures
Supplementary Figures 1–8
Rights and permissions
About this article
Cite this article
Jin, G., Xu, C., Zhang, X. et al. Atad3a suppresses Pink1-dependent mitophagy to maintain homeostasis of hematopoietic progenitor cells. Nat Immunol 19, 29–40 (2018). https://doi.org/10.1038/s41590-017-0002-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-017-0002-1
This article is cited by
-
Establishment and analysis of a novel diagnostic model for systemic juvenile idiopathic arthritis based on machine learning
Pediatric Rheumatology (2024)
-
Leukemia and mitophagy: a novel perspective for understanding oncogenesis and resistance
Annals of Hematology (2024)
-
ATAD3A-related pontocerebellar hypoplasia: new patients and insights into phenotypic variability
Orphanet Journal of Rare Diseases (2023)
-
Targeting ATAD3A-PINK1-mitophagy axis overcomes chemoimmunotherapy resistance by redirecting PD-L1 to mitochondria
Cell Research (2023)
-
Transcription factor Nkx2-3 maintains the self-renewal of hematopoietic stem cells by regulating mitophagy
Leukemia (2023)