Abstract
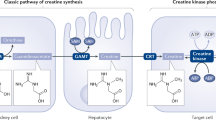
Creatine kinases (CKs) provide local ATP production in periods of elevated energetic demand, such as during rapid anabolism and growth. Thus, creatine energetics has emerged as a major metabolic liability in many rapidly proliferating cancers. Whether CKs can be targeted therapeutically is unknown because no potent or selective CK inhibitors have been developed. Here we leverage an active site cysteine present in all CK isoforms to develop a selective covalent inhibitor of creatine phosphagen energetics, CKi. Using deep chemoproteomics, we discover that CKi selectively engages the active site cysteine of CKs in cells. A co-crystal structure of CKi with creatine kinase B indicates active site inhibition that prevents bidirectional phosphotransfer. In cells, CKi and its analogs rapidly and selectively deplete creatine phosphate, and drive toxicity selectively in CK-dependent acute myeloid leukemia. Finally, we use CKi to uncover an essential role for CKs in the regulation of proinflammatory cytokine production in macrophages.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available as Excel spreadsheets in Supplementary Tables 1–10. Source data are provided with this paper. All additional data is available upon request from the corresponding authors.
References
Kazak, L. & Cohen, P. Creatine metabolism: energy homeostasis, immunity and cancer biology. Nat. Rev. Endocrinol. 16, 421–436 (2020).
Fenouille, N. et al. The creatine kinase pathway is a metabolic vulnerability in EVI1-positive acute myeloid leukemia. Nat. Med. 23, 301–313 (2017).
Loo, J. M. et al. Extracellular metabolic energetics can promote cancer progression. Cell 160, 393–406 (2015).
Maguire, O. A. et al. Creatine-mediated crosstalk between adipocytes and cancer cells regulates obesity-driven breast cancer. Cell Metab. 33, 499–512.e496 (2021).
Zhang, L. et al. Creatine promotes cancer metastasis through activation of Smad2/3. Cell Metab. 33, 1111–1123.e1114 (2021).
Papalazarou, V. et al. The creatine–phosphagen system is mechanoresponsive in pancreatic adenocarcinoma and fuels invasion and metastasis. Nat. Metab. 2, 62–80 (2020).
Streijger, F. et al. Mice lacking brain-type creatine kinase activity show defective thermoregulation. Physiol. Behav. 97, 76–86 (2009).
Streijger, F. et al. Structural and behavioural consequences of double deficiency for creatine kinases BCK and UbCKmit. Behav. Brain Res. 157, 219–234 (2005).
Van der Zee, C. Hypothalamic plasticity of neuropeptide Y is lacking in brain-type creatine kinase double knockout mice with defective thermoregulation. Eur. J. Pharmacol. 719, 137–144 (2013).
Steeghs, K. et al. Cytoarchitectural and metabolic adaptations in muscles with mitochondrial and cytosolic creatine kinase deficiencies. Mol. Cell. Biochem. 184, 183–194 (1998).
Kurth, I. et al. Therapeutic targeting of SLC6A8 creatine transporter suppresses colon cancer progression and modulates human creatine levels. Sci. Adv. 7, eabi7511 (2021).
Kazak, L. et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655 (2015).
Bong, S. M. et al. Structural studies of human brain-type creatine kinase complexed with the ADP–Mg2+–NO3–creatine transition-state analogue complex. FEBS Lett. 582, 3959–3965 (2008).
Shen, Y. Q., Tang, L., Zhou, H. M. & Lin, Z. J. Structure of human muscle creatine kinase. Acta Crystallogr. D Biol. Crystallogr. 57, 1196–1200 (2001).
Fritz-Wolf, K., Schnyder, T., Wallimann, T. & Kabsch, W. Structure of mitochondrial creatine kinase. Nature 381, 341–345 (1996).
Eder, M., Fritz-Wolf, K., Kabsch, W., Wallimann, T. & Schlattner, U. Crystal structure of human ubiquitous mitochondrial creatine kinase. Proteins 39, 216–225 (2000).
Xiao, H. et al. A quantitative tissue-specific landscape of protein redox regulation during aging. Cell 180, 968–983 e924 (2020).
Lu, W. et al. Correction: fragment-based covalent ligand discovery. RSC Chem. Biol. 2, 670–671 (2021).
Backus, K. M. et al. Proteome-wide covalent ligand discovery in native biological systems. Nature 534, 570–574 (2016).
Kuljanin, M. et al. Reimagining high-throughput profiling of reactive cysteines for cell-based screening of large electrophile libraries. Nat. Biotechnol. 39, 630–641 (2021).
Weerapana, E. et al. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468, 790–795 (2010).
Vinogradova, E. V. et al. An activity-guided map of electrophile–cysteine interactions in primary human T cells. Cell 182, 1009–1026.e1029 (2020).
Coles, B. F. & Kadlubar, F. F. Detoxification of electrophilic compounds by glutathione S-transferase catalysis: determinants of individual response to chemical carcinogens and chemotherapeutic drugs? Biofactors 17, 115–130 (2003).
Li, Q. et al. Insights into the phosphoryl transfer mechanism of human ubiquitous mitochondrial creatine kinase. Sci. Rep. 6, 38088 (2016).
Smith, R. A., Hartley, R. C. & Murphy, M. P. Mitochondria-targeted small molecule therapeutics and probes. Antioxid. Redox Signal. 15, 3021–3038 (2011).
Lillie, J. W. et al. Cyclocreatine (1-carboxymethyl-2-iminoimidazolidine) inhibits growth of a broad spectrum of cancer cells derived from solid tumors. Cancer Res. 53, 3172–3178 (1993).
Naito, Y., Takagi, T. & Higashimura, Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch. Biochem. Biophys. 564, 83–88 (2014).
Hudalla, H. et al. Carbonic anhydrase inhibition ameliorates inflammation and experimental pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 61, 512–524 (2019).
Strowitzki, M. J. et al. Carbon dioxide sensing by immune cells occurs through carbonic anhydrase 2-dependent changes in intracellular pH. J. Immunol. 208, 2363–2375 (2022).
Zhang, X. et al. Calcium/calmodulin-dependent protein kinase (CaMK) Ialpha mediates the macrophage inflammatory response to sepsis. J. Leukoc. Biol. 90, 249–261 (2011).
Reddy, A. et al. pH-Gated succinate secretion regulates muscle remodeling in response to exercise. Cell 183, 62–75 (2020).
Johnson, J. L. et al. An atlas of substrate specificities for the human serine/threonine kinome. Nature 613, 759–766 (2023).
Hutti, J. E. et al. A rapid method for determining protein kinase phosphorylation specificity. Nat. Methods 1, 27–29 (2004).
Turk, B. E., Huang, L. L., Piro, E. T. & Cantley, L. C. Determination of protease cleavage site motifs using mixture-based oriented peptide libraries. Nat. Biotechnol. 19, 661–667 (2001).
Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. D Biol. Crystallogr. 66, 133–144 (2010).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Darabedian, N., Chen, T. C., Molina, H., Pratt, M. R. & Schönthal, A. H. Bioorthogonal profiling of a cancer cell proteome identifies a large set of 3-bromopyruvate targets beyond glycolysis. ACS Chem. Biol. 13, 3054–3058 (2018).
Mills, E. L. et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556, 113–117 (2018).
Pettinger, J., Carter, M., Jones, K. & Cheeseman, M. D. Kinetic optimization of lysine-targeting covalent inhibitors of HSP72. J. Med. Chem. 62, 11383–11398 (2019).
Acknowledgements
This work was supported by the Claudia Adams Barr Program (E.T.C.), the Lavine Family Fund (E.T.C.), the Pew Charitable Trust (E.T.C.), National Institutes of Health (NIH) CA259739-01 (E.T.C.), AG071966-01 (E.T.C.), NIH DK123095 (E.T.C.), The Smith Family Foundation (E.T.C.), T32CA236754 (N.D.), the National Cancer Center (NCI) (H.X. and N.D.) NCI R35 CA210030 (K. Stegmaier) and P50 CA206963 (K. Stegmaier), K99AG073461 (H.X.), NCI R35 CA231991 (B.F.C.), the Linde Family Foundation (S.D.-P.), the Doris Duke Charitable Foundation (S.D.-P.), Deerfield 3DC (S.D.-P.), Taiho Pharmaceuticals (S.D.-P.), NCI K99 CA263161 (S.L), and NIH (4R00DK123321-03), Mathers Foundation (MF-2204-02617) and American Cancer Society (DBG-23-983219-01-TBE) (E.L.M). S.L. is a Fellow of the Leukemia & Lymphoma Society. This work was based upon research conducted at the Northeastern Collaborative Access Team beamlines, which were funded by the National Institute of General Medical Sciences from the National Institutes of Health (P30 GM124165). The Eiger 16M detector on the 24-ID-E beamline was funded by a NIH-Office of Research Infrastructure Programs High-End Instrumentation grant (S10OD021527). This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
E.T.C. and N.S.G. conceived of and designed the study. N.D. and S.L. performed cellular experiments and analyzed data. W.J., M.F., J.C. and T.Z. designed and conducted chemical syntheses. N.D. E.M.H, J.L.J. and T.M.Y carried out and analyzed data from mass spectrometry experiments. N.D. assisted with protein expression and purification. H.X. developed the CPT screening platform. E.V.V., K. Senkane and B.F.C. conceived, designed and conducted gel-based screening experiments. E.L.M. performed macrophage experiments and metabolomics experiments. S.D.-P. oversaw CK expression and purification and crystallization. J.C. performed molecular modeling. K. Stegmaier and S.L. contributed to conceptual design and provided cellular reagents. E.T.C. and N.S.G. directed research, oversaw the experiments and wrote the manuscript with assistance from the other authors. L.C.C oversaw kinome profiling experiments.
Corresponding authors
Ethics declarations
Competing interests
N.S.G. is a founder, science advisory board member (SAB) and equity holder in Syros, C4, Allorion, Lighthorse, Voronoi, Inception, Matchpoint, CobroVentures, GSK, Shenandoah (board member), Larkspur (board member) and Soltego (board member). The Gray lab receives or has received research funding from Novartis, Takeda, Astellas, Taiho, Jansen, Kinogen, Arbella, Deerfield, Springworks, Interline and Sanofi. E.T.C. is a founder, board member and equity holder in Matchpoint Therapeutics and Aevum Therapeutics. T.Z. and J.C. are founders, equity holders and consultants in Matchpoint Therapeutics. J.C. is a scientific co-founder M3 Bioinformatics & Technology Inc., and consultant and equity holder for Soltego and Allorion. K. Stegmaier has funding from Novartis Institute of Biomedical Research and Kronos Bio, consults for and has stock options in Auron Therapeutics and previously consulted for Kronos Bio and AstraZeneca. T.M.Y. is a co-founder, stockholder and on the board of directors of DESTROKE, Inc. T.Z., W.J., N.D., N.S.G., J.C. and E.T.C. are inventors on a patent for CK inhibitors WO 2022/087433 A8. All other authors declare no potential conflict of interest. B.F.C. is a founder and scientific advisor to Vividion Therapeutics.
Peer review
Peer review information
Nature Chemical Biology thanks Apirat Chaikuad and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterizing CKi interaction with CKB.
(a) Establishing gel-based screening of recombinant CKB in Ramos cell lysates for engagement of cysteine 283 by competition labeling with iodoacetamide-rhodamine. Single experiment shown. (b) Fragment competition labeling of recombinant CKB in Ramos cell lysates identifies small molecules that compete with iodoacetamide-rhodamine labeling. CKi is highlighted in green. Single experiment shown. (c) Structure of CKi-alkyne. (d) Left: Intact protein MS of 800 nM human recombinant CKB incubated for 2 h with DMSO at 37 °C. Right: Intact protein MS of 800 nM human recombinant CKB incubated for 2 h with 1.6 μM CKi-alkyne at 37 °C. (e) UCSD-AML1 cells incubated with alkyne-CKi followed by conjugation to flouorophore for gel based detection. Fluorophore-conjugated iodoacetamide included as a promiscuous labeling control. (f) Crystal structure active site of human CKB bound to CKi highlighting Cys283, ADP, creatine, location of ligands based on PDB: 3B6R.
Extended Data Fig. 2 Effects of CKi on recombinant CKB.
(a) Electron density of CKi in the active site of CKB. A 2FO-FC map of CKi and its attached residue (Cys283) at sigma 1.0. (b) % CKi engagement with human recombinant CKB assessed by monitoring intact mass of CKB over 2 h at 37 °C. (c) Recombinant CKB phosphotransfer activity ± incubation with CKi for indicated times. n = 3 independent experiments. (d) Kinact/Ki determined using % CKi engagement with human recombinant CKB assessed by monitoring intact mass of CKB over 2 h at 37 °C at different concentrations of CKi. n = 3. (e) ATP/ADP ratio determined in UCSD-AML1 after treating with 0, 1.25, 2.5 5, 10, and 20 μM CKi for 16 h using ADP/ATP ratio assay kit. n = 3 biological cell replicates. (f) Structure of select CKi analogs with increasing hydrocarbon lengths. (g) Recombinant CKB phosphotransfer activity ± 2 h preincubation with CKi analogs with increasing hydrocarbon length. (h) Creatine and phosphocreatine abundance in UCSD-AML1 and A549 cells treated with 10 μM CKi. n = 5. (i) HEK293 cells over-expressing (OE) Flag-CKB exhibit significant increase in total cellular CKB content. Representative blot from 2 independent experiments shown.
Extended Data Fig. 3 Phosphoproteome remodeling induced by CKi and MitoCKi.
(a) The effect of CKi on cell viability in a range of cancer cell lines. HEK; HEK293 cells, HEKOECKB are cells generated in Extended Data Fig. 2i. n = 8. (b) Cell type specific phosphoproteomic signature of UCSD-AML1 and A549 cells treated for 16 h with 10 μM CKi or 5 μM MitoCKi. n = 5. (c) Full phosphoproteomic analysis of UCSD-AML1 or A549 cells treated with 10 μM CKi or 5 μM MitoCKi for 16 h. (n = 5). Upregulated and downregulated sites were then subjected to kinase enrichment analysis as describe in Methods. (d) Volcano plots showing phosphoproteomic analysis of UCSD-AML1 or A549 cells treated with 10 μM CKi or 5 μM MitoCKi for 16 h. n = 5. (e) Volcano plots showing phosphoproteomic analysis of UCSD-AML1 or A549 cells treated with 10 μM CKi or 5 μM MitoCKi for 16 h. n = 5.
Extended Data Fig. 4 CKi flow cytometry analysis.
(a) Flow cytometry analysis using Annexin V and 7-AAD staining of UCSD-AML1 or A549 cells treated with DMSO or 10 μM CKi for 16 h. n = 3. (b) Flow cytometry analysis using Click-iT Plus EdU Alexa Fluor 647 and FxCycle Violet staining of UCSD-AML1 or A549 cells treated with DMSO or 10 μM CKi for 16 h. n = 3.
Extended Data Fig. 5 CKi and MitoCKi flow cytometry analyses.
(a) Flow cytometry analysis using Annexin V and 7-AAD staining of UCSD-AML1 or A549 cells treated with DMSO, 2.5 or 5 μM MitoCKi for 16 h. (b) Quantification of fraction of cells that are apoptotic after 16 h of treatment with DMSO, 2.5 or 5 μM MitoCKi. n = 3. (c) Flow cytometry analysis using Click-iT Plus EdU Alexa Fluor 647 and FxCycle Violet staining of UCSD-AML1 or A549 cells treated with DMSO, 2.5 or 5 μM MitoCKi for 16 h. (d) Quantification of fraction of cells that are in S, G0/G1 and G2/M phase of the cell cycle after 16 h of treatment with DMSO, 2.5 or 5 μM MitoCKi. n = 3.
Extended Data Fig. 6 Kinome analysis and TLR pathway analysis of CKi.
(a) Kinome-wide selectivity profile for compound CKi at 10 μM. CKi was tested at 10 μM on a panel of 468 kinases (Creatine Kinases are not in the panel). The results are displayed as red circles with their sizes correlating with the inhibitor’s binding affinity (percent DMSO control). Complete dataset is included in Supplementary Table 7. (b) Immunoblot of IκBα and phosphorylated IκBα in THP1 cells ± CKi and ± LPS. Representative blot from 3 independent experiments shown.
Supplementary information
Supplementary Information
Supplementary note containing all chemical synthesis information.
Supplementary Tables
Supplementary Tables 1–10.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Uncropped western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig.2
Statistical source data.
Source Data Extended Data Fig. 2
Uncropped western blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Uncropped western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Darabedian, N., Ji, W., Fan, M. et al. Depletion of creatine phosphagen energetics with a covalent creatine kinase inhibitor. Nat Chem Biol 19, 815–824 (2023). https://doi.org/10.1038/s41589-023-01273-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-023-01273-x
This article is cited by
-
Functionalizing tandem mass tags for streamlining click-based quantitative chemoproteomics
Communications Chemistry (2024)