Abstract
Advances in synthetic biology enable microbial hosts to synthesize valuable natural products in an efficient, cost-competitive and safe manner. However, current engineering endeavors focus mainly on enzyme engineering and pathway optimization, leaving the role of cofactors in microbial production of natural products and cofactor engineering largely ignored. Here we systematically engineered the supply and recycling of three cofactors (FADH2, S-adenosyl-l-methion and NADPH) in the yeast Saccharomyces cerevisiae, for high-level production of the phenolic acids caffeic acid and ferulic acid, the precursors of many pharmaceutical molecules. Tailored engineering strategies were developed for rewiring biosynthesis, compartmentalization and recycling of the cofactors, which enabled the highest production of caffeic acid (5.5 ± 0.2 g l−1) and ferulic acid (3.8 ± 0.3 g l−1) in microbial cell factories. These results demonstrate that cofactors play an essential role in driving natural product biosynthesis and the engineering strategies described here can be easily adopted for regulating the metabolism of other cofactors.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the findings of this study are available within the article and its Supplementary Information files. Source data are provided with this paper. Extra data are available from the corresponding author upon request.
Change history
16 August 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41589-022-01129-w
References
Paddon, C. J. et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 496, 528–532 (2013).
Luo, X. Z. et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 567, 123–126 (2019).
Galanie, S., Thodey, K., Trenchard, I. J., Interrante, M. F. & Smolke, C. D. Complete biosynthesis of opioids in yeast. Science 349, 1095–1100 (2015).
Chen, R. B., Yang, S., Zhang, L. & Zhou, Y. J. J. Advanced strategies for production of natural products in yeast. iScience 23, 100879 (2020).
Chen, X. L., Li, S. B. & Liu, L. M. Engineering redox balance through cofactor systems. Trends Biotechnol. 32, 337–343 (2014).
Thomas, D. & Surdin-Kerjan, Y. Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol Mol. Biol. Rev. 61, 503–532 (1997).
Abbas, C. A. & Sibirny, A. A. Genetic control of biosynthesis and transport of riboflavin and flavin nucleotides and construction of robust biotechnological producers. Microbiol Mol. Biol. R. 75, 321 (2011).
Sporty, J. L. et al. Single sample extraction protocol for the quantification of NAD and NADH redox states in Saccharomyces cerevisiae. J. Sep. Sci. 31, 3202–3211 (2008).
Pallotta, M. L. et al. Saccharomyces cerevisiae mitochondria can synthesise FMN and FAD from externally added riboflavin and export them to the extramitochondrial phase. FEBS Lett. 428, 245–249 (1998).
Silva, H. & Lopes, N. M. F. Cardiovascular effects of caffeic acid and its derivatives: a comprehensive review. Front Physiol. 11, 595516 (2020).
Vanholme, R. et al. Caffeoyl shikimate esterase (CSE) is an enzyme in the lignin biosynthetic pathway in Arabidopsis. Science 341, 1103–1106 (2013).
Liu, L. Q. et al. Engineering the biosynthesis of caffeic acid in Saccharomyces cerevisiae with heterologous enzyme combinations. Eng.-Prc. 5, 287–295 (2019).
Phillips, I. R. & Shephard, E. A. Flavin-containing monooxygenases: mutations, disease and drug response. Trends Pharmacol. Sci. 29, 294–301 (2008).
Louie, T. M., Xie, X. S. & Xun, L. Y. Coordinated production and utilization of FADH(2) by NAD(P)H-flavin oxidoreductase and 4-hydroxyphenylacetate 3-monooxygenase. Biochem. 42, 7509–7517 (2003).
Yang, J. Z. et al. Green production of silybin and isosilybin by merging metabolic engineering approaches and enzymatic catalysis. Metab. Eng. 59, 44–52 (2020).
Rodriguez, A., Kildegaard, K. R., Li, M. J., Borodina, I. & Nielsen, J. Establishment of a yeast platform strain for production of p-coumaric acid through metabolic engineering of aromatic amino acid biosynthesis. Metab. Eng. 31, 181–188 (2015).
Wang, J. P. et al. Complete proteomic-based enzyme reaction and inhibition kinetics reveal how monolignol biosynthetic enzyme families affect metabolic flux and lignin in Populus trichocarpa. Plant Cell 26, 894–914 (2014).
Chen, Z. Y., Sun, X. X., Li, Y., Yan, Y. J. & Yuan, Q. P. Metabolic engineering of Escherichia coli for microbial synthesis of monolignols. Metab. Eng. 39, 102–109 (2017).
Liu, Q. et al. Rewiring carbon metabolism in yeast for high level production of aromatic chemicals. Nat. Commun. 10, 4976 (2019).
Yu, T. et al. Reprogramming yeast metabolism from alcoholic fermentation to lipogenesis. Cell 174, 1549–1558 (2018).
Frick, O. & Wittmann, C. Characterization of the metabolic shift between oxidative and fermentative growth in Saccharomyces cerevisiae by comparative 13C flux analysis. Micro. Cell Fact. 4, 30 (2005).
Campos-Bermudez, V. A., Bologna, F. P., Andreo, C. S. & Drincovich, M. F. Functional dissection of Escherichia coli phosphotransacetylase structural domains and analysis of key compounds involved in activity regulation. FEBS J. 277, 1957–1966 (2010).
Bergman, A., Siewers, V., Nielsen, J. & Chen, Y. Functional expression and evaluation of heterologous phosphoketolases in Saccharomyces cerevisiae. Amb. Express 6, 115 (2016).
Mironov, V. N. et al. Functional organization of the riboflavin biosynthesis operon from Bacillus subtilis SHgw. Mol. Gen. Genet. 242, 201–208 (1994).
Zakal’skii, A. E. et al. Cloning of the RIB1 gene coding for the enzyme of the first stage of flavinogenesis in the yeast Pichia guilliermondi, GTP cyclohydrolase, in Escherichia coli cells. Genetika 26, 614–620 (1990).
Mack, M., van Loon, A. P. & Hohmann, H. P. Regulation of riboflavin biosynthesis in Bacillus subtilis is affected by the activity of the flavokinase/flavin adenine dinucleotide synthetase encoded by ribC. J. Bacteriol. 180, 950–955 (1998).
Reihl, P. & Stolz, J. The monocarboxylate transporter homolog Mch5p catalyzes riboflavin (vitamin B2) uptake in Saccharomyces cerevisiae. J. Biol. Chem. 280, 39809–39817 (2005).
Fischer, M. & Bacher, A. Biosynthesis of flavocoenzymes. Nat. Prod. Rep. 22, 324–350 (2005).
Vogl, C. et al. Characterization of riboflavin (vitamin B2) transport proteins from Bacillus subtilis and Corynebacterium glutamicum. J. Bacteriol. 189, 7367–7375 (2007).
Chen, H., Wang, Z., Cai, H. & Zhou, C. Progress in the microbial production of S-adenosyl-l-methionine. World J. Microbiol. Biotechnol. 32, 153 (2016).
Thomas, D. & Surdin-Kerjan, Y. SAM1, the structural gene for one of the S-adenosylmethionine synthetases in Saccharomyces cerevisiae. J. Biol. Chem. 262, 16704–16709 (1987).
Suliman, H. S., Sawyer, G. M., Appling, D. R. & Robertus, J. D. Purification and properties of cobalamin-independent methionine synthase from Candida albicans and Saccharomyces cerevisiae. Arch. Biochem. Biophys. 441, 56–63 (2005).
Murphy, J. T. & Spence, K. D. Transport of S-adenosylmethionine in Saccharomyces cerevisiae. J. Bacteriol. 109, 499–504 (1972).
Gaynor, P. M. & Carman, G. M. Phosphatidylethanolamine methyltransferase and phospholipid methyltransferase activities from Saccharomyces cerevisiae. Enzymological and kinetic properties. Biochim. Biophys. Acta 1045, 156–163 (1990).
Hoffman, D. R., Haning, J. A. & Cornatzer, W. E. Microsomal phosphatidylethanolamine methyltransferase: inhibition by S-adenosylhomocysteine. Lipids 16, 561–567 (1981).
Tehlivets, O., Hasslacher, M. & Kohlwein, S. D. S-adenosyl-l-homocysteine hydrolase in yeast: key enzyme of methylation metabolism and coordinated regulation with phospholipid synthesis. FEBS Lett. 577, 501–506 (2004).
Hoffman, D. R., Marion, D. W., Cornatzer, W. E. & Duerre, J. A. S-Adenosylmethionine and S-adenosylhomocystein metabolism in isolated rat liver. Effects of L-methionine, L-homocystein, and adenosine. J. Biol. Chem. 255, 10822–10827 (1980).
Lecoq, K., Belloc, I., Desgranges, C. & Daignan-Fornier, B. Role of adenosine kinase in Saccharomyces cerevisiae: identification of the ADO I gene and study of the mutant phenotypes. Yeast 18, 335–342 (2001).
Gerber, A., Grosjean, H., Melcher, T. & Keller, W. Tad1p, a yeast tRNA-specific adenosine deaminase, is related to the mammalian pre-mRNA editing enzymes ADAR1 and ADAR2. EMBO J. 17, 4780–4789 (1998).
Xavier, K. B. & Bassler, B. L. LuxS quorum sensing: more than just a numbers game. Curr. Opin. Microbiol. 6, 191–197 (2003).
Kunjapur, A. M., Hyun, J. C. & Prather, K. L. Deregulation of S-adenosylmethionine biosynthesis and regeneration improves methylation in the E. coli de novo vanillin biosynthesis pathway. Micro. Cell Fact. 15, 61 (2016).
Perl, M., Kearney, E. B. & Singer, T. P. Transport of riboflavin into yeast cells. J. Biol. Chem. 251, 3221–3228 (1976).
Wang, M., Chen, B., Fang, Y. & Tan, T. Cofactor engineering for more efficient production of chemicals and biofuels. Biotechnol. Adv. 35, 1032–1039 (2017).
Minard, K. I. & McAlister-Henn, L. Sources of NADPH in yeast vary with carbon source. J. Biol. Chem. 280, 39890–39896 (2005).
Li, S., Li, Y. & Smolke, C. D. Strategies for microbial synthesis of high-value phytochemicals. Nat. Chem. 10, 395–404 (2018).
Cao, X., Yang, S., Cao, C. & Zhou, Y. J. Harnessing sub-organelle metabolism for biosynthesis of isoprenoids in yeast. Synth. Syst. Biotechnol. 5, 179–186 (2020).
Hammer, S. K. & Avalos, J. L. Harnessing yeast organelles for metabolic engineering. Nat. Chem. Biol. 13, 823–832 (2017).
Huang, Y. et al. Enhanced S-adenosyl-l-methionine production in Saccharomyces cerevisiae by spaceflight culture, overexpressing methionine adenosyltransferase and optimizing cultivation. J. Appl. Microbiol. 112, 683–694 (2012).
Heo, K. T., Kang, S. Y. & Hong, Y. S. De novo biosynthesis of pterostilbene in an Escherichia coli strain using a new resveratrol O-methyltransferase from Arabidopsis. Micro. Cell Fact. 16, 30 (2017).
Kuras, L., Cherest, H., Surdin-Kerjan, Y. & Thomas, D. A heteromeric complex containing the centromere binding factor 1 and two basic leucine zipper factors, Met4 and Met28, mediates the transcription activation of yeast sulfur metabolism. EMBO J. 15, 2519–2529 (1996).
Kuras, L., Barbey, R. & Thomas, D. Assembly of a bZIP-bHLH transcription activation complex: formation of the yeast Cbf1-Met4-Met28 complex is regulated through Met28 stimulation of Cbf1 DNA binding. EMBO J. 16, 2441–2451 (1997).
Li, Y. et al. De novo biosynthesis of caffeic acid from glucose by engineered Saccharomyces cerevisiae. ACS Synth. Biol. 9, 756–765 (2020).
Zhou, P. et al. Metabolic engineering of Saccharomyces cerevisiae for enhanced production of caffeic acid. Appl. Microbiol. Biotechnol. 105, 5809–5819 (2021).
Lin, Y. & Yan, Y. Biosynthesis of caffeic acid in Escherichia coli using its endogenous hydroxylase complex. Micro. Cell Fact. 11, 42 (2012).
Huang, Q., Lin, Y. & Yan, Y. Caffeic acid production enhancement by engineering a phenylalanine over-producing Escherichia coli strain. Biotechnol. Bioeng. 110, 3188–3196 (2013).
Yang, S., Cao, X., Yu, W., Li, S. & Zhou, Y. J. Efficient targeted mutation of genomic essential genes in yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 104, 3037–3047 (2020).
Zhou, Y. J. et al. Modular pathway engineering of diterpenoid synthases and the mevalonic acid pathway for miltiradiene production. J. Am. Chem. Soc. 134, 3234–3241 (2012).
Mikkelsen, M. D. et al. Microbial production of indolylglucosinolate through engineering of a multi-gene pathway in a versatile yeast expression platform. Metab. Eng. 14, 104–111 (2012).
Mans, R. et al. CRISPR/Cas9: a molecular Swiss army knife for simultaneous introduction of multiple genetic modifications in Saccharomyces cerevisiae. FEMS Yeast Res. 15, fov004 (2015).
Verduyn, C., Postma, E., Scheffers, W. A. & Van Dijken, J. P. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8, 501–517 (1992).
Sinha, T., Makia, M., Du, J., Naash, M. I. & Al-Ubaidi, M. R. Flavin homeostasis in the mouse retina during aging and degeneration. J. Nutr. Biochem. 62, 123–133 (2018).
Lu, H. et al. A consensus S. cerevisiae metabolic model Yeast8 and its ecosystem for comprehensively probing cellular metabolism. Nat. Commun. 10, 3586 (2019).
Heirendt, L. et al. Creation and analysis of biochemical constraint-based models using the COBRA Toolbox v.3.0. Nat. Protoc. 14, 639–702 (2019).
Acknowledgements
This work was funded by the National Key Research and Development Program of China (grant no. 2018YFA0900300 to Y.J.Z.), National Natural Science Foundation of China (grant nos. 31970316 to L.Z., 21922812 to Y.J.Z. and 32000231 to R.C.), LiaoNing Revitalization Talents Program (grant no. XLYC1807191 to Y.J.Z.) and Shanghai Sail Program (grant no. 19YF1459300 to R.C.). We appreciate Y. Zhao (East China University of Science and Technology) for helpful discussion about FAD quantification. We also thank J. Yang and C. Song for raw data summary and AiMi Academic Services (www.aimieditor.com) for English language editing.
Author information
Authors and Affiliations
Contributions
Y.J.Z. and L.Z., conceived the original research. R.C designed and performed experiments and analyzed the data. R.C., J.G., X.Z. and W.Y. performed the fed-batch fermentations. R.C. and X.C performed the metabolic analysis. Y.C. performed the MFA. R.C., L.Z. and Y.J.Z. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Authors have filed two patents (PCT/CN2021/138511 and PCT/CN2021/138510) for protection of the cofactor engineering strategies of the work described herein.
Peer review
Peer review information
Nature Chemical Biology thanks Rodrigo Ledesma-Amaro and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
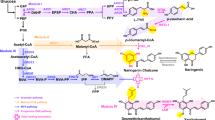
Extended Data Fig. 1 Biosynthesis of phenolic acids and their derivatives.
Structures of pCA- (green), CaA- (orange) and FA-derived (pink) natural products, and their pharmaceutical properties.
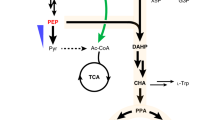
Extended Data Fig. 3 Engineering the biosynthesis of precursor pCA in yeast.
a. Overview of the engineered metabolic pathway for pCA biosynthesis. Optimization of the shikimic acid and aromatic amino acid (AAA) pathways together with the introduction of bacteria- and plant-derived phenolic acid pathways were used for pCA production. ARO10 encoding phenylpyruvate decarboxylase, PDC5 encoding pyruvate decarboxylase, ARO4 encoding 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP) synthase, ARO7 encoding chorismate mutase, EcAROL encoding E. coli shikimate kinase II, FjTAL encoding Flavobacterium johnsoniae tyrosine ammonia lyase, SbPAL1 encoding Isatis indigotica phenylalanine ammonia lyase 1, AtCPR1 encoding Arabidopsis thaliana cytochrome P450 reductase, PtrC4H1/2 encoding Populus trichocarpa cinnamic acid hydroxylase 1/2, PtrC3H encoding P. trichocarpa coumarate-3-hydroxylase, CYP complex consisting of PtrC4H1 + PtrC4H2 + PtrC3H. PEP, phosphoenolpyruvate; DHQ, 3-dehydroquinate; DHS, 3-dehydro-shikimate; SHIK, shikimate; S3P, shikimate-3-phosphate; EPSP, 5-enolpyruvyl-shikimate-3-phosphate; CHA, chorismic acid; PPA, prephenate; PPY, phenylpyruvate; HPP, para-hydroxy-phenylpyruvate; Phe, phenylalanine; Tyr, tyrosine; CA, cinnamic acid; pCA, coumaric acid; CaA, caffeic acid; FA, ferulic acid. b. Production of pCA in yeast. c. Introduction of the CYP complex further improved pCA biosynthesis. All data represent the mean of n = 3 independent biological samples and error bars show standard deviation. All data represent the mean of n = 3 independent biological samples and error bars show standard deviation. Statistical analysis was performed by using Student’s t test (one-tailed; two-sample unequal variance; *p < 0.05, **p < 0.01, ***p < 0.001).
Extended Data Fig. 4 Optimization of HPAB/C and the shikimic acid pathway for CaA production.
a. Overview of the yeast metabolic pathway for CaA biosynthesis. ARO1 encoding shikimate dehydrogenase, ARO2 encoding chorismate synthase, ARO3 encoding DAHP synthase, MtPDH1 encoding Medicago truncatula prephenate dehydrogenase, PHA2 encoding S. cerevisiae prephenate dehydratase 2. See Supplementary Fig. 5 legend regarding other abbreviations. The fusion EcHPAB-EcHPAC (a) and PaHPAB-SeHPAC (b) result in a negative effect on CaA production. PaHPAB, encoding Pseudomonas aeruginosa HpaB; SeHPAC, encoding Salmonella enterica HpaC; EcHPAB: encoding E. coli HpaB; EcHPAC: encoding E. coli HpaC. d. Further optimization of the shikimic acid pathway increased CaA titers with the CYP system. Cells were grown in defined minimal medium containing 20 g/L glucose and cultures were extracted after 96 h of growth for phenolic acid detection. All data represent the mean of n = 3 independent biological samples and error bars show standard deviation. All data represent the mean of n = 3 independent biological samples and error bars show standard deviation. Statistical analysis was performed by using Student’s t test (one-tailed; two-sample unequal variance; *p < 0.05, **p < 0.01, ***p < 0.001).
Extended Data Fig. 5 Effect of combining MCH5 overexpression with FADH2 strategies on CaA production.
a. Schematic illustration of the combinations of FADH2 strategies. See Fig. 4 legend regarding abbreviations. (+) indicates expression under strong constitutive promoters. Strain RB19 is the reference strain for regulating FADH2. b. Relative titers of engineered strains normalized to the titers of reference strain RB19. All data represent the mean of n = 3 independent biological samples and error bars show standard deviation.
Extended Data Fig. 6 Quantification of flavin derived cofactors in CaA producing strains.
a. Representative chromatographs of standards and samples. Total concentration of intracellular FMN(H2) (b), intracellular riboflavin (c) and extracellular riboflavin (d) was detected at different time points. μmol gDCW-1 refers to the number of moles of cofactor per gram of dry cell weight (DCW). All strains were grown at 30 °C in 20 mL of Delft-D medium containing 20 g/L glucose with an initial OD600 of 0.1. All data represent the mean of n = 3 independent biological samples and error bars show standard deviation. Statistical analysis was performed by using Student’s t test (one-tailed; two-sample unequal variance; *p < 0.05, **p < 0.01, ***p < 0.001).
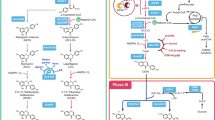
Extended Data Fig. 8 Cofactor engineering and pathway optimization for FA production.
a. Schematic illustration of the SAM cycle. LiMETK1 encoding Leishmania infantum S-adenosylmethionine synthetases, MET6 encoding S. cerevisiae methionine synthase, cMTHFR encoding chemeric methylenetetrahydrofolate reductase. SAM, S-adenosylmethionine; Met, methionine; Hcys, homocysteine; SAH, S-adenosylhomocysteine; CH3-THF, 5-methyl tetrahydrogen folic acid; 5, 10-CH2-THF, 5, 10-methylene tetrahydrogen folic acid; ATP, adenosine triphosphate. b. Overexpression of the SAM pathway reduced phenolic acid titers. c. Enhancing CaA biosynthesis improved FA production. ARO1 encoding shikimate dehydrogenase, ARO2 encoding chorismate synthase, ARO3 encoding DAHP synthase, MtPDH1 encoding Medicago truncatula prephenate dehydrogenase, PHA2 encoding S. cerevisiae prephenate dehydratase 2, LmXFPK, encoding Leuconostoc mesenteroides phosphoketolase; CkPTA, encoding Clostridium kluyveri phosphotransacetylase. gpp1Δ indicates deletion of GPP1 (encoding GAP phosphatase). d. Overexpression of NtCOMT1 using multiple gene copies under of the control of PGAL or strong constitutive promoters improved FA production and the CaA conversion rate. The # indicates expression under strong constitutive promoters (PtHXT7 and PTDH3). All data represent the mean of n = 3 independent biological samples and error bars show standard deviation. All data represent the mean of n = 3 independent biological samples and error bars show standard deviation. Statistical analysis was performed by using Student’s t test (one-tailed; two-sample unequal variance; *p < 0.05, **p < 0.01, ***p < 0.001).
Extended Data Fig. 9 Quantification of cellular SAM and SAH in FA producing strains.
a. Representative chromatographs of standards and samples. Corresponding mass spectra of SAM (b) and SAH (c). d. Cellular SAM/SAH ratios were detected at different time points. All strains were grown at 30 °C in 20 mL of Delft-D medium containing 20 g/L glucose. All data represent the mean of n = 3 independent biological samples and error bars show standard deviation. Statistical analysis was performed by using Student’s t test (one-tailed; two-sample unequal variance; *p < 0.05, **p < 0.01, ***p < 0.001).
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, Tables 1–5 and data legends.
Supplementary Data 1
Primers used in this study.
Supplementary Data 2
Overview of DNA constructs used in this study.
Supplementary Data 3
Codon-optimized genes used in this study.
Supplementary Data 4
Homology sequences for integration at selected chromosomal loci.
Supplementary Data 5
New reactions for metabolic flux analysis.
Supplementary Data 6
Code of culture simulation for metabolic flux analysis.
Supplementary Data 7
Code of yeast genome-scale metabolic model for metabolic flux analysis.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, R., Gao, J., Yu, W. et al. Engineering cofactor supply and recycling to drive phenolic acid biosynthesis in yeast. Nat Chem Biol 18, 520–529 (2022). https://doi.org/10.1038/s41589-022-01014-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-022-01014-6
This article is cited by
-
Exploring the role of flavin-dependent monooxygenases in the biosynthesis of aromatic compounds
Biotechnology for Biofuels and Bioproducts (2024)
-
Minimal aromatic aldehyde reduction (MARE) yeast platform for engineering vanillin production
Biotechnology for Biofuels and Bioproducts (2024)
-
De novo biosynthesis of the hops bioactive flavonoid xanthohumol in yeast
Nature Communications (2024)
-
Modular assembly of an artificially concise biocatalytic cascade for the manufacture of phenethylisoquinoline alkaloids
Nature Communications (2024)
-
Combinatorial optimization of gene expression through recombinase-mediated promoter and terminator shuffling in yeast
Nature Communications (2024)