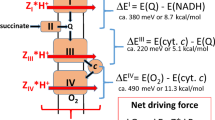
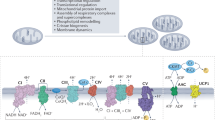
Abstract
Metabolites once considered solely in catabolism or anabolism turn out to have key regulatory functions. Among these, the citric acid cycle intermediate succinate stands out owing to its multiple roles in disparate pathways, its dramatic concentration changes and its selective cell release. Here we propose that succinate has evolved as a signaling modality because its concentration reflects the coenzyme Q (CoQ) pool redox state, a central redox couple confined to the mitochondrial inner membrane. This connection is of general importance because CoQ redox state integrates three bioenergetic parameters: mitochondrial electron supply, oxygen tension and ATP demand. Succinate, by equilibrating with the CoQ pool, enables the status of this central bioenergetic parameter to be communicated from mitochondria to the rest of the cell, into the circulation and to other cells. The logic of this form of regulation explains many emerging roles of succinate in biology, and suggests future research questions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Martínez-Reyes, I. & Chandel, N. S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 11, 102 (2020).
Murphy, M. P. & O’Neill, L. A. J. Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell 174, 780–784 (2018).
Winther, S., Trauelsen, M. & Schwartz, T. W. Protective succinate-SUCNR1 metabolic stress signaling gone bad. Cell Metab. 33, 1276–1278 (2021).
Chandel, N. S. Evolution of mitochondria as signaling organelles. Cell Metab. 22, 204–206 (2015).
Nicholls, D. G. & Ferguson, S. J. Bioenergetics 3rd edn, 31–55 (Academic Press, 2003).
Majmundar, A. J., Wong, W. J. & Simon, M. C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40, 294–309 (2010).
Mihaylova, M. M. & Shaw, R. J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 13, 1016–1023 (2011).
Efeyan, A., Comb, W. C. & Sabatini, D. M. Nutrient-sensing mechanisms and pathways. Nature 517, 302–310 (2015).
Stefely, J. A. & Pagliarini, D. J. Biochemistry of mitochondrial coenzyme Q biosynthesis. Trends Biochem. Sci. 42, 824–843 (2017).
Lester, R. L., Crane, F. L. & Hatefi, Y. Coenzyme Q: a new group of quinones. J. Am. Chem. Soc. 80, 4751–4752 (1958).
Rebstock, A. S., Mongin, F., Trecourt, F. & Queguiner, G. Metallation of pyridines and quinolines in the presence of a remote carboxylate group. New syntheses of heterocyclic quinones. Org. Biomol. Chem. 2, 291–295 (2004).
Wang, Y. & Hekimi, S. Understanding ubiquinone. Trends Cell Biol. 26, 367–378 (2016).
Rich, P. R. & Maréchal, A. The mitochondrial respiratory chain. Essays Biochem. 47, 1–23 (2010).
Mitchell, P. Protonmotive redox mechanism of the cytochrome b-c1 complex in the respiratory chain: protonmotive ubiquinone cycle. FEBS Lett. 56, 1–6 (1975).
Walker, J. E. The ATP synthase: the understood, the uncertain and the unknown. Biochem. Soc. Trans. 41, 1–16 (2013).
Sun, F. et al. Crystal structure of mitochondrial respiratory membrane protein complex II. Cell 121, 1043–1057 (2005).
Burger, N. et al. A sensitive mass spectrometric assay for mitochondrial CoQ pool redox state in vivo. Free Radic. Biol. Med. 147, 37–47 (2020).
Zhang, J. et al. Accumulation of succinate in cardiac ischemia primarily occurs via canonical krebs cycle activity. Cell Rep. 23, 2617–2628 (2018).
Reddy, A. et al. pH-gated succinate secretion regulates muscle remodeling in response to exercise. Cell 183, 62–75.e17 (2020). This paper demonstrated protonation-dependent secretion of succinate via MCT1.
Chouchani, E. T. et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435 (2014).
Kumar, R. et al. A redox cycle with complex II prioritizes sulfide quinone oxidoreductase dependent H2S oxidation. J. Biol. Chem. 298, 101435 (2021).
Spinelli, J. B. et al. Fumarate is a terminal electron acceptor in the mammalian electron transport chain. Science 374, 1227–1237 (2021).
Mills, E. L. et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560, 102–106 (2018).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013). This paper defined succinate accumulation as an upstream metabolite regulator of inflammatory cytokine production.
Murphy, M. P. How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 (2009).
Hinkle, P. C., Butow, R. A., Racker, E. & Chance, B. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XV. Reverse electron transfer in the flavin-cytochrome b region of the respiratory chain of beef heart submitochondrial particles. J. Biol. Chem. 242, 5169–5173 (1967).
Kussmaul, L. & Hirst, J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Natl Acad. Sci. USA 103, 7607–7612 (2006).
Hirst, J., King, M. S. & Pryde, K. R. The production of reactive oxygen species by complex I. Biochem. Soc. Trans 36, 976–980 (2008). This paper illustrated the mechanism of superoxide production by mitochondrial complex I at the FMN site.
Agip, A.-N. A., Blaza, J. N., Fedor, J. G. & Hirst, J. Mammalian respiratory complex I through the lens of cryo-EM. Annu. Rev. Biophys. 48, 165–184 (2019).
Pryde, K. R. & Hirst, J. Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer. J. Biol. Chem. 286, 18056–18065 (2011).
Goncalves, R. L., Quinlan, C. L., Perevoshchikova, I. V., Hey-Mogensen, M. & Brand, M. D. Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J. Biol. Chem. 290, 209–227 (2015).
Chance, B. & Hollunger, G. The interaction of energy and electron transfer reactions in mitochondria. I. General properties and nature of the products of succinate-linked reduction of pyridine nucleotide. J. Biol. Chem. 236, 1534–1543 (1961).
Krishnamoorthy, G. & Hinkle, P. C. Studies on the electron transfer pathway, topography of iron-sulfur centers, and site of coupling in NADH-Q oxidoreductase. J. Biol. Chem. 263, 17566–17575 (1988).
Cino, M. & Del Maestro, R. F. Generation of hydrogen peroxide by brain mitochondria: the effect of reoxygenation following postdecapitative ischemia. Arch. Biochem. Biophys. 269, 623–638 (1989).
Lambert, A. J. & Brand, M. D. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I). J. Biol. Chem. 279, 39414–39420 (2004).
Yin, Z. et al. Structural basis for a complex I mutation that blocks pathological ROS production. Nat. Commun. 12, 707 (2021).
Tyler, D. D. Polarographic assay and intracellular distribution of superoxide dismutase in rat liver. Biochemical J. 147, 493–504 (1975).
Sena, L. A. & Chandel, N. S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 48, 158–167 (2012).
Cox, A. G., Winterbourn, C. C. & Hampton, M. B. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem. J. 425, 313–325 (2009).
Reczek, C. R. & Chandel, N. S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 33, 8–13 (2015).
Holmstrom, K. M. & Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 15, 411–421 (2014).
Murphy, M. P. et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 13, 361–366 (2011).
Lennicke, C. & Cochemé, H. M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 81, 3691–3707 (2021).
Brand, M. D. et al. Suppressors of superoxide-H2O2 production at site IQ of mitochondrial complex I protect against stem cell hyperplasia and ischemia-reperfusion injury. Cell Metab. 24, 582–592 (2016).
Laukka, T. et al. Fumarate and succinate regulate expression of hypoxia-inducible genes via TET enzymes. J. Biol. Chem. 291, 4256–4265 (2016).
Mills, E. L. et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167, 457–470.e13 (2016). This paper defined succinate accumulation as an upstream metabolite regulator of inflammatory cytokine production via mitochondrial ROS production.
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
Muzik, O., Mangner, T. J., Leonard, W. R., Kumar, A. & Granneman, J. G. Sympathetic innervation of cold-activated brown and white fat in lean young adults. J. Nucl. Med. 58, 799–806 (2017).
Lettieri Barbato, D. et al. Glutathione decrement drives thermogenic program in adipose cells. Sci. Rep. 5, 13091 (2015).
Ro, S. H. et al. Sestrin2 inhibits uncoupling protein 1 expression through suppressing reactive oxygen species. Proc. Natl Acad. Sci. USA 111, 7849–7854 (2014).
Chouchani, E. T. et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 532, 112–116 (2016).
Han, Y. H. et al. Adipocyte-specific deletion of manganese superoxide dismutase protects from diet-induced obesity through increased mitochondrial uncoupling and biogenesis. Diabetes 65, 2639–2651 (2016).
Schneider, K. et al. Increased energy expenditure, Ucp1 expression, and resistance to diet-induced obesity in mice lacking nuclear factor-erythroid-2-related transcription factor-2 (Nrf2). J. Biol. Chem. 291, 7754–7766 (2016).
Lee, S. J., Kim, S. H., Park, K. M., Lee, J. H. & Park, J. W. Increased obesity resistance and insulin sensitivity in mice lacking the isocitrate dehydrogenase 2 gene. Free Radic. Biol. Med. 99, 179–188 (2016).
Chouchani, E. T., Kazak, L. & Spiegelman, B. M. Mitochondrial reactive oxygen species and adipose tissue thermogenesis: bridging physiology and mechanisms. J. Biol. Chem. 292, 16810–16816 (2017).
Kazak, L. et al. UCP1 deficiency causes brown fat respiratory chain depletion and sensitizes mitochondria to calcium overload-induced dysfunction. Proc. Natl Acad. Sci. USA 114, 7981–7986 (2017).
Sanchez-Alavez, M., Bortell, N., Galmozzi, A., Conti, B. & Marcondes, M. C. Reactive oxygen species scavenger N-acetyl cysteine reduces methamphetamine-induced hyperthermia without affecting motor activity in mice. Temperature 1, 227–241 (2014).
Sanchez-Alavez, M. et al. ROS and sympathetically mediated mitochondria activation in brown adipose tissue contribute to methamphetamine-induced hyperthermia. Front. Endocrinol. 4, 44 (2013).
Jedrychowski, M. P. et al. Facultative protein selenation regulates redox sensitivity, adipose tissue thermogenesis, and obesity. Proc. Natl Acad. Sci. USA 117, 10789–10796 (2020).
Mills, E. L. et al. Cysteine 253 of UCP1 regulates energy expenditure and sex-dependent adipose tissue inflammation. Cell Metab. 34, 140–157.e8 (2022).
Xiao, H. et al. A quantitative tissue-specific landscape of protein redox regulation during aging. Cell 180, 968–983.e24 (2020).
Fernández-Agüera, M. C. et al. Oxygen sensing by arterial chemoreceptors depends on mitochondrial complex I signaling. Cell Metab. 22, 825–837 (2015).
Harman, D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11, 298–300 (1956).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Ristow, M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat. Med. 20, 709–711 (2014).
Schulz, T. J. et al. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 6, 280–293 (2007).
Scialò, F. et al. Mitochondrial ROS produced via reverse electron transport extend animal lifespan. Cell Metab. 23, 725–734 (2016). This paper demonstrates a role for regulated production of mitochondrial ROS in organismal lifespan.
Yang, W. & Hekimi, S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 8, e1000556 (2010).
Owusu-Ansah, E., Song, W. & Perrimon, N. Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell 155, 699–712 (2013).
Fiermonte, G. et al. The sequence, bacterial expression, and functional reconstitution of the rat mitochondrial dicarboxylate transporter cloned via distant homologs in yeast and Caenorhabditis elegans. J. Biol. Chem. 273, 24754–24759 (1998).
Losman, J.-A., Koivunen, P. & Kaelin, W. G. 2-Oxoglutarate-dependent dioxygenases in cancer. Nat. Rev. Cancer 20, 710–726 (2020).
Selak, M. A. et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 7, 77–85 (2005). This paper illustrated the importance of SDH mutations in cance pathogenesis via accumulated succinate.
Carey, B. W., Finley, L. W., Cross, J. R., Allis, C. D. & Thompson, C. B. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518, 413–416 (2015).
Hems, R., Stubbs, M. & Krebs, H. A. Restricted permeability of rat liver for glutamate and succinate. Biochem. J. 107, 807–815 (1968).
Ehinger, J. K. et al. Cell-permeable succinate prodrugs bypass mitochondrial complex I deficiency. Nat. Commun. 7, 12317 (2016).
MacDonald, M. J., Fahien, L. A., Mertz, R. J. & Rana, R. S. Effect of esters of succinic acid and other citric acid cycle intermediates on insulin release and inositol phosphate formation by pancreatic islets. Arch. Biochem. Biophys. 269, 400–406 (1989).
Hochachka, P. W. & Dressendorfer, R. H. Succinate accumulation in man during exercise. Eur. J. Appl. Physiol. Occup. Physiol. 35, 235–242 (1976).
Taegtmeyer, H. Metabolic responses to cardiac hypoxia. Increased production of succinate by rabbit papillary muscles. Circ. Res. 43, 808–815 (1978).
Hochachka, P. W., Owen, T. G., Allen, J. F. & Whittow, G. C. Multiple end products of anaerobiosis in diving vertebrates. Comp. Biochem. Physiol. B 50, 17–22 (1975).
Prag, H. A. et al. Mechanism of succinate efflux upon reperfusion of the ischaemic heart. Cardiovasc. Res. 117, 1188–1201 (2020).
Bisbach, C. M. et al. Succinate can shuttle reducing power from the hypoxic retina to the O2-rich pigment epithelium. Cell Rep. 31, 107606 (2020).
Wu, J.-Y. et al. Cancer-derived succinate promotes macrophage polarization and cancer metastasis via succinate receptor. Mol. Cell 77, 213–227.e5 (2020).
Mills, E. L. et al. UCP1 governs liver extracellular succinate and inflammatory pathogenesis. Nat. Metab. 3, 604–617 (2021).
An, Y. A. et al. The mitochondrial dicarboxylate carrier prevents hepatic lipotoxicity by inhibiting white adipocyte lipolysis. J. Hepatol. 75, 387–399 (2021).
Andrienko, T. N., Pasdois, P., Pereira, G. C., Ovens, M. J. & Halestrap, A. P. The role of succinate and ROS in reperfusion injury—a critical appraisal. J. Mol. Cell. Cardiol. 110, 1–14 (2017).
Bisbach, C., Hass, D. & Hurley, J. Monocarboxylate transporter 1 (MCT1) mediates succinate export in the retina. Preprint at bioRxiv https://doi.org/10.1101/2021.11.19.469314 (2021).
Pajor, A. M. Sodium-coupled dicarboxylate and citrate transporters from the SLC13 family. Pflug. Arch. 466, 119–130 (2014).
Acknowledgements
This work was supported by the Claudia Adams Barr Program (E.T.C.), the Lavine Family Fund (E.T.C.), the Pew Charitable Trust (E.T.C.), NIH DK123095 (E.T.C.), The Smith Family Foundation (E.T.C.), Medical Research Council UK (MC_UU_00015/3) (M.P.M.) and by a Wellcome Trust Investigator award (220257/Z/20/Z) (M.P.M.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
E.T.C. is a founder, board member and equity holder in Matchpoint Therapeutics. M.P.M. holds shares in Antipodean Pharmaceuticals Inc. E.T.C. and M.P.M. hold patents in the field of therapeutic modulation of succinate metabolism.
Peer review
Peer review information
Nature Chemical Biology thanks Navdeep Chandel, Luke O’Neill and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Murphy, M.P., Chouchani, E.T. Why succinate? Physiological regulation by a mitochondrial coenzyme Q sentinel. Nat Chem Biol 18, 461–469 (2022). https://doi.org/10.1038/s41589-022-01004-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-022-01004-8