Abstract
Native porphyran is a hybrid of porphryan and agarose. As a common element of edible seaweed, this algal galactan is a frequent component of the human diet. Bacterial members of the human gut microbiota have acquired polysaccharide utilization loci (PULs) that enable the metabolism of porphyran or agarose. However, the molecular mechanisms that underlie the deconstruction and use of native porphyran remains incompletely defined. Here, we have studied two human gut bacteria, porphyranolytic Bacteroides plebeius and agarolytic Bacteroides uniformis, that target native porphyran. This reveals an exo-based cycle of porphyran depolymerization that incorporates a keystone sulfatase. In both PULs this cycle also works together with a PUL-encoded agarose depolymerizing machinery to synergistically reduce native porphyran to monosaccharides. This provides a framework for understanding the deconstruction of a hybrid algal galactan, and insight into the competitive and/or syntrophic relationship of gut microbiota members that target rare nutrients.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Structure coordinates and structure factors determined in this study have been deposited in the PDB with the accession codes of 7LHA, 7LJ2, 7LJJ, 7LK7, 7LNP, 5T98, 5T99 and 7LH6. Existing structure coordinates used in this study that are already deposited in the PDB are 2WVU, 1YNP, 5T9G, 7CWD, 4PSR, 5K9H, 4ZRX, 3GZA, 4OUE, 1ODU, 3UET and 2G2V. All other data are available by request.
References
Ndeh, D. et al. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature 544, 65–70 (2017).
Cuskin, F. et al. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature 517, 165–169 (2015).
Kloareg, B. & Quatrano, R. S. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Annu. Rev. 26, 259–315 (1988).
Hehemann, J.-H., Boraston, A. B. & Czjzek, M. A sweet new wave: structures and mechanisms of enzymes that digest polysaccharides from marine algae. Curr. Opin. Struct. Biol. 28, 77–86 (2014).
Ficko-Blean, E. et al. Carrageenan catabolism is encoded by a complex regulon in marine heterotrophic bacteria. Nat. Commun. 8, 1685 (2017).
Hettle, A. G. et al. Insights into the κ/ι-carrageenan metabolism pathway of some marine Pseudoalteromonas species. Commun. Biol. 2, 474 (2019).
Reisky, L. et al. A marine bacterial enzymatic cascade degrades the algal polysaccharide ulvan. Nat. Chem. Biol. 15, 803–812 (2019).
Wells, M. L. et al. Algae as nutritional and functional food sources: revisiting our understanding. J. Appl. Phycol. 29, 949–982 (2017).
Hehemann, J.-H. et al. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 464, 908–912 (2010).
Pluvinage, B. et al. Molecular basis of an agarose metabolic pathway acquired by a human intestinal symbiont. Nat. Commun. 9, 1043 (2018).
Mathieu, S. et al. Ancient acquisition of ‘alginate utilization loci’ by human gut microbiota. Sci. Rep. 8, 8075 (2018).
Thomas, F., Hehemann, J.-H., Rebuffet, E., Czjzek, M. & Michel, G. Environmental and gut bacteroidetes: the food connection. Front. Microbiol. https://doi.org/10.3389/fmicb.2011.00093 (2011).
Pudlo, N. A. et al. Extensive transfer of genes for edible seaweed digestion from marine to human gut bacteria. Preprint at bioRxiv https://doi.org/10.1101/2020.06.09.142968 (2020).
Shepherd, E. S., DeLoache, W. C., Pruss, K. M., Whitaker, W. R. & Sonnenburg, J. L. An exclusive metabolic niche enables strain engraftment in the gut microbiota. Nature 557, 434–438 (2018).
Kearney, S. M., Gibbons, S. M., Erdman, S. E. & Alm, E. J. Orthogonal dietary niche enables reversible engraftment of a gut bacterial commensal. Cell Rep. 24, 1842–1851 (2018).
Rinaudo, M. in Comprehensive Glycoscience 691–735 (Elsevier, 2007).
Rees, D. Enzymic synthesis of 3:6-anhydro-l-galactose within porphyran from l-galactose 6-sulphate units. Biochem. J. 81, 347–352 (1961).
Rees, D. Enzymic desulphation of porphyran. Biochem. J. 80, 449–453 (1961).
Correc, G., Hehemann, J.-H., Czjzek, M. & Helbert, W. Structural analysis of the degradation products of porphyran digested by Zobellia galactanivorans β-porphyranase A. Carbohydr. Polym. 83, 277–283 (2011).
Hehemann, J.-H., Kelly, A. G., Pudlo, N. A., Martens, E. C. & Boraston, A. B. Bacteria of the human gut microbiome catabolize red seaweed glycans with carbohydrate-active enzyme updates from extrinsic microbes. Proc. Natl Acad. Sci. USA 109, 19786–19791 (2012).
Hehemann, J. H., Smyth, L., Yadav, A., Vocadlo, D. J. & Boraston, A. B. Analysis of a keystone enzyme in agar hydrolysis provides insight into the degradation of a polysaccharide from red seaweeds. J. Biol. Chem. 287, 13985–13995 (2012).
Hobbs, M. E., Williams, H. J., Hillerich, B., Almo, S. C. & Raushel, F. M. l-Galactose metabolism in Bacteroides vulgatus from the human gut microbiota. Biochemistry 53, 4661–4670 (2014).
Marquardt, T. et al. High-resolution crystal structure of AKR11C1 from Bacillus halodurans: an NADPH-dependent 4-hydroxy-2,3-trans-nonenal reductase. J. Mol. Biol. 354, 304–316 (2005).
Barbeyron, T. et al. Matching the diversity of sulfated biomolecules: creation of a classification database for sulfatases reflecting their substrate specificity. PLoS ONE 11, e0164846 (2016).
Lombard, V., Golaconda Ramulu, H., Drula, E., Coutinho, P. M. & Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 42, D490–D495 (2014).
Bond, C. S. et al. Structure of a human lysosomal sulfatase. Structure 5, 277–289 (1997).
Hettle, A. G. et al. The molecular basis of polysaccharide sulfatase activity and a nomenclature for catalytic subsites in this class of enzyme. Structure 26, 747–758.e4 (2018).
Cartmell, A. et al. How members of the human gut microbiota overcome the sulfation problem posed by glycosaminoglycans. Proc. Natl Acad. Sci. USA 114, 7037–7042 (2017).
Hehemann, J.-H. et al. Single cell fluorescence imaging of glycan uptake by intestinal bacteria. ISME J. 13, 1883–1889 (2019).
Reintjes, G., Arnosti, C., Fuchs, B. M. & Amann, R. An alternative polysaccharide uptake mechanism of marine bacteria. ISME J. 11, 1640–1650 (2017).
Larsbrink, J. et al. A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes. Nature 506, 498–502 (2014).
Lee, C. H. et al. A novel agarolytic β-galactosidase acts on agarooligosaccharides for complete hydrolysis of agarose into monomers. Appl. Environ. Microbiol. 80, 5965–5973 (2014).
Robb, C. S., Reisky, L., Bornscheuer, U. T. & Hehemann, J.-H. Specificity and mechanism of carbohydrate demethylation by cytochrome P450 monooxygenases. Biochem. J. 475, 3875–3886 (2018).
Reisky, L. et al. Oxidative demethylation of algal carbohydrates by cytochrome P450 monooxygenases. Nat. Chem. Biol. 14, 342–344 (2018).
Grondin, J. M., Tamura, K., Déjean, G., Abbott, D. W. & Brumer, H. Polysaccharide utilization loci: fueling microbial communities. J. Bacteriol. 199, e00860-16 (2017).
Luis, A. S. et al. Dietary pectic glycans are degraded by coordinated enzyme pathways in human colonic Bacteroides. Nat. Microbiol. 3, 210–219 (2018).
Gasteiger, E. et al. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31, 3784–3788 (2003).
Jones, D. R. et al. Analysis of active site architecture and reaction product linkage chemistry reveals a conserved cleavage substrate for an endo-alpha-mannanase within diverse yeast mannans. J. Mol. Biol. 432, 1083–1097 (2020).
Stevenson, T. T. & Furneaux, R. H. Chemical methods for the analysis of sulphated galactans from red algae. Carbohydr. Res. 210, 277–298 (1991).
Voiges, K., Adden, R., Rinken, M. & Mischnick, P. Critical re-investigation of the alditol acetate method for analysis of substituent distribution in methyl cellulose. Cellulose 19, 993–1004 (2012).
Patankar, M. S., Oehninger, S., Barnett, T., Williams, R. L. & Clark, G. F. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 268, 21770–21776 (1993).
Heiss, C., Wang, Z. & Azadi, P. Sodium hydroxide permethylation of heparin disaccharides. Rapid Commun. Mass Spectrom. 25, 774–778 (2011).
Kariya, Y. et al. Preparation of completely 6-O-desulfated heparin and its ability to enhance activity of basic fibroblast growth factor. J. Biol. Chem. 275, 25949–25958 (2000).
Ceroni, A. et al. GlycoWorkbench: A tool for the computer-assisted annotation of mass spectra of glycans. J. Proteome Res. 7, 1650–1659 (2008).
Nielsen, S. S. in Food Analysis Laboratory Manual 137–141 (Springer, 2017).
Robb, M., Hobbs, J. K. & Boraston, A. B. Separation and visualization of glycans by fluorophore-assisted carbohydrate electrophoresis. Methods Mol. Biol. 1588, 215–221 (2017). in.
Abbott, D. W. & Boraston, A. B. Quantitative approaches to the analysis of carbohydrate-binding module function. Methods Enzymol. 510, 211–231 (2012).
Tomme, P., Boraston, A., Kormos, J. M., Warren, R. A. J. & Kilburn, D. G. Affinity electrophoresis for the identification and characterization of soluble sugar binding by carbohydrate-binding modules. Enzym. Microb. Technol. 27, 453–458 (2000).
Masuko, T. et al. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 339, 69–72 (2005).
Rogowski, A. et al. Glycan complexity dictates microbial resource allocation in the large intestine. Nat. Commun. 6, 7481 (2015).
Powell, H. R. The Rossmann Fourier autoindexing algorithm in MOSFLM. Acta Cryst. 55, 1690–1695 (1999).
Jones, P. et al. InterProScan 5: genome-scale protein function classification. Bioinformatics 30, 1236–1240 (2014).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Mccoy, A. J. et al. Phaser crystallographic software. J. Appl. Cryst. 40, 658–674 (2007).
Lammerts van Bueren, A. et al. Analysis of the reaction coordinate of alpha-l-fucosidases: a combined structural and quantum mechanical approach. J. Am. Chem. Soc. 132, 1804–1806 (2010).
Cowtan, K. The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr. D. Biol. Crystallogr. 62, 1002–1201 (2006).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D. Biol. Crystallogr. 66, 486–501 (2010).
Murshudov, G. N. et al. REFMAC 5 for the refinement of macromolecular crystal structures. Acta Crystallogr. Sect. D. Biol. Crystallogr. 67, 355–367 (2011).
Brünger, A. T. Free R value: a novel statistical quantity for assessing the accuracy of crystal structures. Nature 355, 472–475 (1992).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. Sect. D. Biol. Crystallogr. 66, 12–21 (2010).
Acknowledgements
This research was supported by Natural Sciences and Engineering Research Council of Canada Discovery Grants (nos. FRN 04355 to A.B.B. and FRN 03929 to W.F.Z.), funding from Agriculture and Agri-Food Canada (project grant no. J-002262 to D.W.A.) and Beef and Cattle Research Council (grant no. FOS.04.17 / J-001973 to D.W.A.). G.R. was supported by funding from the European Union’s Horizons 2020 research and innovations program under the Marie Skłodowska-Curie grant agreement no. 840804. We thank the staff at the CLS where diffraction data were collected. The CLS is supported by the Natural Sciences and Engineering Research Council of Canada, the National Research Council of Canada, the Canadian Institutes of Health Research, the Province of Saskatchewan, Western Economic Diversification Canada and the University of Saskatchewan.
Author information
Authors and Affiliations
Contributions
C.S.R and A.B.B. initiated the project. A.B.B. and D.W.A directed the project. C.S.R performed structural analyses of BuS1_11 and BpGH29 and activity analyses of the S1_11 and GH29 enzymes. J.K.H. performed all galactose release assays for the enzymes in this study and kinetic assays of BpLGDH. B.P. determined the structures of BuGH2A. G.R. and G.G. performed glycan uptake studies. L.K., S.M. and C.A. performed growth assays. C.V. determined the structure of BpLGDH. A.G.H. performed kinetic analysis of BpS1_11. R.H. performed binding studies of SusE-like proteins. N., X.X., T.M. and W.F.Z. performed carbohydrate analysis by NMR, GC–MS and LC–MS. A.B.B. wrote the paper with input from C.S.R. and D.W.A. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Uwe Bornscheuer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
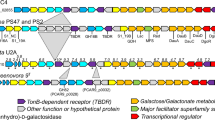
Extended Data Fig. 1 Structures of algal galactans and the polysaccharide utilization loci (PUL) that target them.
The chemical structures of a, agarose, b, porphyran, and c, native porphyran comprising blocks of porphyran and agarose. d, The PorPUL is shown on top with the AgaPUL beneath. Individual genes are labelled by general function according to the provided legend. Glycoside hydrolases with known agarose and porphyran activity have blue and green labels, respectively. Black labels indicate gene products whose activity are identified in this study. Gray labels indicate unknowns. See also Supplementary Table 1.
Extended Data Fig. 2 Activity analysis of BpLGDH.
a, screen of BpLGDH activity against various monosaccharides using NAD+ as a co-factor. n = 3 independent reactions were performed with each replicate shown along with the mean ± standard deviation. b and c show the kinetics using NAD+ and NADP+, respectively, as a co-factor when excess L-galactose is used as a substrate. d, the kinetics of L-galactose oxidation using excess NADP+ as a co-factor. The data is shown as the mean ± standard deviation of n = 3 independent reactions (see Supplementary Fig. 1 for independent data points). The solid line shows the best fit line to the Michaelis-Menton equation.
Extended Data Fig. 3 Structural analysis of BpLGDH.
a, structure of the BpLGDH dimer with one monomer shown in cartoon and the other as a solvent accessible surface. The putative catalytic histidine is shown in blue. b, overlap of BpLGDH (blue) with AKR11C1 from Bacillus halodurans (grey, PDB ID 1YNP). c, overlap of the BpLGDH (blue) active site with the active site of AKR11C1 (grey). Conserved residues, including the putative catalytic histidine (H119 in AKR11C1), are shown as sticks. In panels c and d the NADPH bound to AKR11C1 is shown in stick representation.
Extended Data Fig. 4 Kinetic and structural analysis of BuS1_11.
a, kinetic analysis of neoporphyrabiose hydrolysis by BpS1_11*. The data is shown as the mean ± standard deviation of n = 3 independent reactions (see Supplementary Fig. 2 for independent data points). The solid line shows the best fit line to the Michaelis-Menton equation. b, the overall fold of BpS1_11 shown as a cartoon. The bound calcium ion is shown as a green sphere and key residues in the active site are shown as blue sticks. c, electron density maps of NP2 (yellow sticks) bound to the BuS1_11 active site are shown with the 2Fo-Fc map at 1σ in blue (top) and the Fo-Fc omit map at 3σ in green (bottom). d, the solvent accessible surface of the active site is shown in transparent grey with the bound NP2 as yellow sticks. The subsites of the active site are labeled in red according to the nomenclature of Hettle et al.27. e, structural overlap of BuS1_11 (blue) with BT4656 (grey, 5G2V). NP2 is shown as yellow sticks and 2-N,6-O-disulfo-d-glucosamine bound to BT4656 shown as green sticks. Relevant inserted structural motifs in each protein that contribute to carbohydrate specificity are shown as solid cartoons.
Extended Data Fig. 5 Structural analysis of BpGH29.
a, cartoon representation of uncomplexed BpGH29. b, electron density maps of L-galactose (yellow sticks) bound to the BpGH29 active site are shown with the 2Fo-Fc map at 1σ in blue (top) and the Fo-Fc omit map at 3σ in green (bottom). c, the specific interactions between the active site and L-galactose. The nucleophile (N) and acid/base (A/B) are colored blue and organge respectively. d, cartoon representation of BpGH29 in complex with L-galactose. In panels a and c the sidechain proposed to act as the nucleophile (D264) is shown as blue sticks, the sidechain proposed to act as the acid/base in orange sticks, and mobile loops that help form the active site in orange and blue. e, electron density maps of pNP-α-L-galactopyranoside (yellow sticks) bound to the BpGH29D264N active site are shown with the 2Fo-Fc map at 1σ in blue (top) and the Fo-Fc omit map at 3σ in green (bottom). f, pNP-α-L-galactopyranoside bound to the BpGH29 D264N active site shown with the solvent accessible surface of the active site shown in grey.
Extended Data Fig. 6 Enzymatic activity and sequencing of a purified tetrasaccharide derived from porphyran.
The enzyme combinations are indicated on the left with the amounts of d-galactose and l-galactose release displayed as a bar chart. The sites of bond hydrolysis are indicated in the schematics on the right side of the figure. Thirteen nanomoles (13 nmoles) of tetrasaccharide were used in each reaction and each reaction was performed in independent triplicate (n = 3) reactions with each replicate shown along with the mean ± standard deviation.
Extended Data Fig. 7 Structural analysis of BuGH2A.
a, cartoon representation of BuGH2A showing the central (α/β)8-barrel sitting in a nest of four Ig-like domains. b, electron density maps of galactoisofagomine (green sticks) bound to the BuGH2A active site are shown with the 2Fo-Fc map at 1σ in blue (top) and the Fo-Fc omit map at 3σ in green (bottom).
Supplementary information
Supplementary Information
Supplementary Figs. 1–16, Tables 1–8 and References.
Rights and permissions
About this article
Cite this article
Robb, C.S., Hobbs, J.K., Pluvinage, B. et al. Metabolism of a hybrid algal galactan by members of the human gut microbiome. Nat Chem Biol 18, 501–510 (2022). https://doi.org/10.1038/s41589-022-00983-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-022-00983-y
This article is cited by
-
Fungal β-glucan-facilitated cross-feeding activities between Bacteroides and Bifidobacterium species
Communications Biology (2023)
-
Synthesis of 2′-fucosyllactose from apple pomace–derived xyloglucan oligosaccharides by an α-L-fucosidase from Pedobacter sp. CAU209
Applied Microbiology and Biotechnology (2023)
-
Agarolytic Pathway in the Newly Isolated Aquimarina sp. Bacterial Strain ERC-38 and Characterization of a Putative β-agarase
Marine Biotechnology (2023)
-
Sulfated glycan recognition by carbohydrate sulfatases of the human gut microbiota
Nature Chemical Biology (2022)