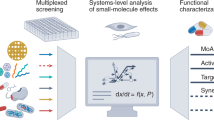
Abstract
Molecular profiling of small molecules offers invaluable insights into the function of compounds and allows for hypothesis generation about small-molecule direct targets and secondary effects. However, current profiling methods are limited in either the number of measurable parameters or throughput. Here we developed a multiplexed, unbiased framework that, by linking genetic to drug-induced changes in nearly a thousand metabolites, allows for high-throughput functional annotation of compound libraries in Escherichia coli. First, we generated a reference map of metabolic changes from CRISPR interference (CRISPRi) with 352 genes in all major essential biological processes. Next, on the basis of the comparison of genetic changes with 1,342 drug-induced metabolic changes, we made de novo predictions of compound functionality and revealed antibacterials with unconventional modes of action (MoAs). We show that our framework, combining dynamic gene silencing with metabolomics, can be adapted as a general strategy for comprehensive high-throughput analysis of compound functionality from bacteria to human cell lines.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article as Supplementary Data. A detailed description of all data analysis steps is published in this article (Methods and Supplementary Information). Matlab code is available for download at https://www.imsb.ethz.ch/research/zampieri-group/resources.html. Metabolome data and results from data analysis can be found as Supplementary Datasets. The MS proteomics raw data and analysis results have been deposited through the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD024133. Genome sequence data have been deposited through SRA BioProject under accession number PRJNA776168 (also see Supplementary Notes). Source data are provided with this paper.
Change history
08 April 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41589-022-01028-0
References
Tacconelli, E. et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 18, 318–327 (2018).
Cox, G. et al. A common platform for antibiotic dereplication and adjuvant discovery. Cell Chem. Biol. 24, 98–109 (2017).
Eckhardt, M., Hultquist, J. F., Kaake, R. M., Hüttenhain, R. & Krogan, N. J. A systems approach to infectious disease. Nat. Rev. Genet. 21, 339–354 (2020).
Imai, Y. et al. A new antibiotic selectively kills Gram-negative pathogens. Nature 576, 459–464 (2019).
Johnson, E. O. et al. Large-scale chemical–genetics yields new M. tuberculosis inhibitor classes. Nature 571, 72–78 (2019).
Mechanism matters. Nat. Med. 16, 347 (2010).
Campos, A. I. & Zampieri, M. Metabolomics-driven exploration of the chemical drug space to predict combination antimicrobial therapies. Mol. Cell 74, 1291–1303. (2019).
Nonejuie, P., Burkart, M., Pogliano, K. & Pogliano, J. Bacterial cytological profiling rapidly identifies the cellular pathways targeted by antibacterial molecules. Proc. Natl Acad. Sci. USA 110, 16169–16174 (2013).
Stokes, J. M. et al. A multiplexable assay for screening antibiotic lethality against drug-tolerant bacteria. Nat. Methods 16, 303–306 (2019).
French, S., Coutts, B. E. & Brown, E. D. Open-source high-throughput phenomics of bacterial promoter-reporter strains. Cell Syst. 7, 339–346 (2018).
Gardner, T. S., Bernardo, D., di, Lorenz, D. & Collins, J. J. Inferring genetic networks and identifying compound mode of action via expression profiling. Science 301, 102–105 (2003).
Zampieri, M. et al. High-throughput metabolomic analysis predicts mode of action of uncharacterized antimicrobial compounds. Sci. Transl. Med. 10, eaal3973 (2018).
Zampieri, M. From the metabolic profiling of drug response to drug mode of action. Curr. Opin. Syst. Biol. 10, 26–33 (2018).
Iorio, F. et al. Discovery of drug mode of action and drug repositioning from transcriptional responses. Proc. Natl Acad. Sci. USA 107, 14621–14626 (2010).
O’Rourke, A. et al. Mechanism-of-action classification of antibiotics by global transcriptome profiling. Antimicrob. Agents Chemother. 64, e01207-19 (2020).
Brötz-Oesterhelt, H., Bandow, J. E. & Labischinski, H. Bacterial proteomics and its role in antibacterial drug discovery. Mass Spectrom. Rev. 24, 549–565 (2005).
Vincent, I. M., Ehmann, D. E., Mills, S. D., Perros, M. & Barrett, M. P. Untargeted metabolomics to ascertain antibiotic modes of action. Antimicrob. Agents Chemother. 60, 2281–2291 (2016).
Fuhrer, T., Heer, D., Begemann, B. & Zamboni, N. High-throughput, accurate mass metabolome profiling of cellular extracts by flow injection-time-of-flight mass spectrometry. Anal. Chem. 83, 7074–7080 (2011).
Mülleder, M. et al. Functional metabolomics describes the yeast biosynthetic regulome. Cell 167, 553–565 (2016).
Sarvin, B. et al. Fast and sensitive flow-injection mass spectrometry metabolomics by analyzing sample-specific ion distributions. Nat. Commun. 11, 3186 (2020).
Qi, L. S. et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013).
Peters, J. M. et al. A comprehensive, CRISPR-based functional analysis of essential genes in bacteria. Cell 165, 1493–1506 (2016).
Silvis, M. R. et al. Morphological and transcriptional responses to CRISPRi knockdown of essential genes in Escherichia coli. mBio 12, e0256121 (2021).
Belenky, P. et al. Bactericidal antibiotics induce toxic metabolic perturbations that lead to cellular damage. Cell Rep. 13, 968–980 (2015).
Zampieri, M., Zimmermann, M., Claassen, M. & Sauer, U. Nontargeted metabolomics reveals the multilevel response to antibiotic perturbations. Cell Rep. 19, 1214–1228 (2017).
Donati, S. et al. Multi-omics analysis of CRISPRi-knockdowns identifies mechanisms that buffer decreases of enzymes in E. coli metabolism. Cell Syst. 12, 56–67 (2021).
Fuhrer, T., Zampieri, M., Sévin, D. C., Sauer, U. & Zamboni, N. Genomewide landscape of gene–metabolome associations in Escherichia coli. Mol. Syst. Biol. 13, 907 (2017).
Tatusov, R. L., Galperin, M. Y., Natale, D. A. & Koonin, E. V. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 28, 33–36 (2000).
You, C. et al. Coordination of bacterial proteome with metabolism by cyclic AMP signalling. Nature 500, 301–306 (2013).
Costanzo, M. et al. The genetic landscape of a cell. Science 327, 425–431 (2010).
de Wet, T. J., Winkler, K. R., Mhlanga, M., Mizrahi, V. & Warner, D. F. Arrayed CRISPRi and quantitative imaging describe the morphotypic landscape of essential mycobacterial genes. eLife 9, e60083 (2020).
Gupta, R., China, A., Manjunatha, U. H., Ponnanna, N. M. & Nagaraja, V. A complex of DNA gyrase and RNA polymerase fosters transcription in Mycobacterium smegmatis. Biochem. Biophys. Res. Commun. 343, 1141–1145 (2006).
Nayar, A. S. et al. Novel antibacterial targets and compounds revealed by a high-throughput cell wall reporter assay. J. Bacteriol. 197, 1726–1734 (2015).
Spitzer, M. et al. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol. Syst. Biol. 7, 499 (2011).
Amaral, L. & Lorian, V. Effects of chlorpromazine on the cell envelope proteins of Escherichia coli. Antimicrob. Agents Chemother. 35, 1923–1924 (1991).
Feng, Y. et al. Global analysis of protein structural changes in complex proteomes. Nat. Biotechnol. 32, 1036–1044 (2014).
Guo, Y. et al. Tail-anchored inner membrane protein elab increases resistance to stress while reducing persistence in Escherichia coli. J. Bacteriol. 199, e00057-17 (2017).
Hu, P. et al. Global functional atlas of Escherichia coli encompassing previously uncharacterized proteins. PLoS Biol. 7, e1000096 (2009).
Lorenz, C., Dougherty, T. J. & Lory, S. Transcriptional responses of Escherichia coli to a small-molecule inhibitor of LolCDE, an essential component of the lipoprotein transport pathway. J. Bacteriol. 198, 3162–3175 (2016).
Říha, M. et al. Novel method for rapid copper chelation assessment confirmed low affinity of d-penicillamine for copper in comparison with trientine and 8-hydroxyquinolines. J. Inorg. Biochem. 123, 80–87 (2013).
Imlay, J. A. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat. Rev. Microbiol. 11, 443–454 (2013).
Macomber, L. & Imlay, J. A. The iron–sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl Acad. Sci. USA 106, 8344–8349 (2009).
Torres-Cabassa, A. S. & Gottesman, S. Capsule synthesis in Escherichia coli K-12 is regulated by proteolysis. J. Bacteriol. 169, 981–989 (1987).
Hao, Z. et al. The multiple antibiotic resistance regulator MarR is a copper sensor in Escherichia coli. Nat. Chem. Biol. 10, 21–28 (2014).
Kirn, T. J., Onyeaso, E., Syed, M. & Weinstein, M. P. Systematic evaluation of commercial susceptibility testing methods for determining the in vitro activity of daptomycin versus Staphylococcus aureus and Enterococci. J. Clin. Microbiol. 52, 1877–1882 (2014).
Peters, J. M. et al. Enabling genetic analysis of diverse bacteria with Mobile-CRISPRi. Nat. Microbiol. 4, 244–250 (2019).
Stokes, J. M. et al. A deep learning approach to antibiotic discovery. Cell 180, 688–702 (2020).
Dubuis, S., Ortmayr, K. & Zampieri, M. A framework for large-scale metabolome drug profiling links coenzyme A metabolism to the toxicity of anti-cancer drug dichloroacetate. Commun. Biol. 1, 101 (2018).
Storey, J. D. A direct approach to false discovery rates. J. R. Stat. Soc. Ser. B 64, 479–498 (2002).
Maier, L. et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555, 623–628 (2018).
Orth, J. D. et al. A comprehensive genome-scale reconstruction of Escherichia coli metabolism–2011. Mol. Syst. Biol. 7, 535 (2011).
Okuda, S. & Yoshizawa, A. C. ODB: a database for operon organizations, 2011 update. Nucleic Acids Res. 39, D552–D555 (2011).
Konig, R. et al. A probability-based approach for the analysis of large-scale RNAi screens. Nat. Methods 4, 847–849 (2007).
Zampieri, M. et al. Metabolic constraints on the evolution of antibiotic resistance. Mol. Syst. Biol. 13, 917 (2017).
Manfredi, P. et al. Defining proteomic signatures to predict multidrug persistence in Pseudomonas aeruginosa. Methods Mol. Biol. 2357, 161–175 (2021).
Zaslaver, A. et al. A comprehensive library of fluorescent transcriptional reporters for Escherichia coli. Nat. Methods 3, 623–628 (2006).
Fang, X. et al. Global transcriptional regulatory network for Escherichia coli robustly connects gene expression to transcription factor activities. Proc. Natl Acad. Sci. USA 114, 10286–10291 (2017).
Wishart, D. S. et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 46, D608–D617 (2018).
Brunk, E. et al. Recon3D enables a three-dimensional view of gene variation in human metabolism. Nat. Biotechnol. 36, 272–281 (2018).
Faith, J. J. et al. Large-scale mapping and validation of Escherichia coli transcriptional regulation from a compendium of expression profiles. PLoS Biol. 5, e8 (2007).
Acknowledgements
We thank U. Sauer for supporting this work and providing laboratory facilities, N. de Souza for helpful feedback and discussions, the Gross and K.C. Huang groups at UCSF and Stanford for discussions and sharing the E. coli CRISPRi library ahead of publication and T.J. de Wet and D.F. Warner at the University of Cape Town for sharing the CRISPRi mutants in M. smegmatis. This work was supported by an NCCR AntiResist project funding to M.Z., U.J. and P.P. (180541); P.P. and L.G. were additionally funded by the European Research Council (grant agreement number 866004), EPIC-XS, project number 823839, funded by the Horizon 2020 programme of the European Union and through a Personalized Health and Related Technologies grant (PHRT-506). C.M. and M.B. were supported by NIH grant AI133191.
Author information
Authors and Affiliations
Contributions
M.Z. designed the project. M.A.-G. and G.H. performed the metabolic profiling of the E. coli mutant library. A.I.C. performed the drug metabolome profiles. K.O. performed the metabolic profiling in human cell lines. C.M. performed the metabolic profiling of M. smegmatis under the supervision of M.B. P.M. performed the genome sequencing and proteome profiling of resistant strains under the supervision of U.J. L.G. performed the limited proteolysis experiments and analyses under the supervision of P.P. M.A.-G., G.H. and M.Z. performed data analysis. All authors contributed to preparing the manuscript.
Corresponding author
Ethics declarations
Competing interests
P.P. is an advisor at Biognosys AG (Schlieren, Switzerland), the company that licenses the LiP technology.
Peer review
Peer review information
Nature Chemical Biology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Metabolome profiles of CRISPRi mutant library.
A) Distribution of significant metabolic changes per gene knockdowns/time points. On average, 24 metabolites underwent significant changes (absolute Z-score ≥ 1 and p value ≤ 1e−5) per mutant and time point, and most of the metabolites (95%) exhibited a significant response in at least one gene knockdown. B) Distribution of significant changes for each metabolite across knockdowns/time points. C) Relative distribution of collected time points per gene knockdown. D) Distribution of Optical Densities (OD600) at sampling. E) Distribution of locality scores for essential (blue) and non-essential knockdowns (red). F) Distribution of the number of significant affected metabolites by essential (blue) and non-essential (red) gene knockdowns in each time point. G) Estimates of growth rates of CRISPRi mutants grown in M9 glucose + 1 mM IPTG. Each dot corresponds to mean ± standard deviation (SD) across three biological replicates. Average Wild-type growth rate ± SD are represented by solid and dashed red lines, respectively. Each dot is color-coded according to the significance in growth rate difference with respect to wild-type (that is t-test analysis). Pie chart reports on the fraction of knockdown strains growing significantly slower than wild type (pvalue ≤ 0.01). H) Median absolute Z-score of significantly affected metabolites (|Z-score|≥ 1 and p value ≤ 1e − 5) in each mutant/time point. Median values for each timepoint across all mutant strains are used to fit a sigmoidal curve (red curve). The blue shaded area identifies the confidence region (95% confidence interval). Green dashed lines report the 95% confidence interval for the time necessary to reach 90% of the maximum estimated changes (that is plateau).
Extended Data Fig. 2 Analysis of growth phenotypes.
A) Growth dynamics of each individual mutant. Each line corresponds to the OD averaged across 3 biological replicates. In orange, mutants exhibiting a sudden drop in OD are highlighted. B) Median absolute Z-score of significantly affected metabolites in each mutant/time point vs estimated growth rate. As expected the slower the growth rate the larger is the impact of gene knockdowns on metabolism7, although the dependency is mild: Spearman correlation of -0.19. C) Distribution of growth rates for essential and non-essential gene knockdowns. D) Distribution of growth rates for gene knockdowns across KEGG pathways.
Extended Data Fig. 3 Metabolome-based predictions of functional gene-gene similarity.
A) ROC analysis comparing the performance of different similarity metrics in detecting pair of genes that are either encoding for subunits of the same protein complex or for iso-enzymes. We used previously published data27 profiling the metabolome across 3873 gene knockout mutants. We selected only genes encoding for proteins complexes and isoenzymes with at least one significant metabolic changes as defined in27 (|Z-score| >5). Area Under the Curve (AUC) is reported for Spearman correlation, mutual information, context likelihood of relatedness (CLR)60 applied on mutual information, iterative similarity and CLR applied on iterative similarity (iSim). The best performance was obtained with iSim (Table S1). To cope with the fact that similarity metrics, like mutual information, can be biased by hidden global patterns in the data (in our case likely to reflect indirect and general type of effects (for example growth rates)), and to take into account the typical patterns of interaction of multivariate datasets, the authors in60 developed an effective and simple approach to normalize pair wise mutual information. The CLR algorithm applies an adaptive background correction step to the matrix of pair-wise similarity scores to eliminate indirect global similarities between drug/gene knockdown metabolome profiles. After computing the similarity between drug/gene pairs, the algorithm compares the similarity between drug/gene A and drug/gene B to the background distribution of similarity scores calculated for all possible drug/gene pairs that include either A or B. The pairs with the most probable functional associations are those whose similarity scores is larger than the background distribution of similarity scores. This step, when applied on mutual information, improves predictions by eliminating ‘promiscuous’ cases, where one gene weakly co-varies with a large numbers of genes. The improvement of CLR applied on iSim are minor. Hence, in this work iSim is used instead of iSim+CLR. B) For each gene, we ranked gene-gene metabolic similarity and performed KEGG-functional enrichment analysis -that is KEGG pathways that exhibit a significant (qvalue ≤ 0.01) enrichment of gene knockdowns exhibiting similar metabolic profiles. In blue the ROC curve obtained by considering only similarities between gene pairs from different operons. In purple, we report ROC analysis of KEGG functional enrichment without accounting for operon structure. D) Each corresponds to a KEGG metabolic pathway and the respective AUC values estimated from gene-gene similarity. Only KEGG pathways with an AUC ≥ 0.6 are reported.
Extended Data Fig. 4 Metabolic profiles of genetic perturbations in Mycobacterium Smegmatis.
A) Growth dynamics of each individual M. smegmatis CRISPR mutant after ATC induction. Each line and errorbar corresponds to the OD600 average and standard deviation across 3 biological replicates. B) Heatmap of metabolome profiles 24 hours after CRISPR induction.
Extended Data Fig. 5 Metabolic profiles of chemical perturbations in A549 cancer cells.
A) Dynamic monitoring of cell confluence (% well area covered by cells) in A549 cancer cell line in the presence of sgRNAs targeting three different sections of thymidylate synthase, in addition to a control sgRNA inducing cell death (lethal control, gLethal) by targeting multiple sections in the genome. B) Dynamic monitoring of cell confluence in A549 cancer cell line treated with different drugs. C) The volcano plots illustrate metabolic changes (that is log2 fold-change and p-value significance) for 1593 putatively annotated metabolites in A549 cells 48 hours after treatment with 14 different drugs. Pvalues are estimated by two-sided t-test analysis. 3 drugs are targeting TYMS (methotrexate, pemetrexed and 5-fluorouracil). The remaining drugs act on largely different cellular processes, such as central metabolism (oxamate, dichloroacetate, carbonyl cyanide, 2-deoxyglucose, UK5099), nucleotide biosynthesis (mercaptopurine), mevalonate pathway (atorvastatin), DNA repair (camptothecin, cisplatin, doxorubicin) and dopamine receptors (trifluoperazine).
Extended Data Fig. 6 Gene-drug associations.
A) Disk diffusion assay testing the susceptibility to ofloxacin (10 μg) in wild-type E. coli and pyrG knockdown. Violin plot represents mean ± SD across 3 biological replicates. Two representative petri dishes are reported on the lower panel. B) Imipramine gene-drug associations. Volcano plot of similarities between drug and knockdowns time dependent metabolic profiles. Notably, we found genes exhibiting more rare but strong associations to chemically induced metabolic changes, such as the association between the gene mediating chromosome partitioning during division mukF, and imipramine, a drug typically used as antidepressant, potentially hinting at the toxic side effects of imipramine as DNA damaging agent; C) Vatalanib gene-drug associations. Vatalanib, a tyrosine kinase inhibitor that interferes with the ATP-binding site of vascular endothelial growth factor receptors (VEGFR1-3), induces similar metabolic changes to purA knockdown, a key gene in the adenosine ribonucleotides de novo biosynthesis. D) fraction of compounds inhibiting at least 50% of the growth-rate at 100 µM for (i) compounds within the top 1% of drug-gene knockdown similarities, (ii) compounds within the top 1% of drug-gene knockout similarities (data reported in27), (iii) all tested compounds. We found that drugs with strong similar metabolic profiles to at least one essential gene knockdown (that is top 1% of all drug-genes associations) are significantly enriched (pvalue = 2.3071e-183 ≤ 0.001 ***, from 13% to 60%) for drugs inducing Growth Inhibition (GI) greater than 50%. On the contrary when selecting the top 1% of similarities between drugs and the ~3800 non-essential gene knockout7,27, we found no significant enrichment and the majority of drugs exhibits relatively low growth inhibition (GI < 50%). E-F-G) Volcano plot of similarities between tegaserod, suloctidil, thiethylperazine and the remaining 1341 drugs. Pvalues are estimated by hypergeometric test analysis. H-J-K) Volcano plot of proteome changes in three tegaserod resistant strains (Table 1 of the main text) with respect to the ancestor wild-type E. coli (Table S7). Proteins with significant (qvalue ≤ 0.05 and |FC|≥ 1) changes with respect to the wild-type are highlighted in red. Pvalues are estimated by two-sided t-test analysis and corrected for multiple tests.
Extended Data Fig. 7 Chloroxine induce copper toxicity.
A-B-C-D) Results from limited proteolysis analysis. Each dot in the volcano plot represents the relative difference in peptide abundance between the treated and untreated proteome extracts. None of the 13797 detected peptides exhibit significant (pvalue < 7.2480e-07, Bonferroni corrected) conformational changes between untreated and whole proteome extracts treated with 4 different concentrations of chloroxine (Table S6). Pvalues are estimated by two-sided t-test analysis. E) Relative growth rates at different concentrations of chloramphenicol with respect to the untreated condition. Wild type E. coli was grown in LB medium with and without 200 µM of CuCl2. Growth inhibitory activity for different chloramphenicol concentrations were measured in triplicates. Errorbars are mean ± standard deviation across three biological replicates. F) 2-Isopropylmaleate z-score levels across the 1342 tested compounds. G) Average Z-score across metabolites in the branched chain amino acids biosynthesis pathway as defined in KEGG. Significance (that is pvalue estimated by permutation test) of enrichment analysis is testing for each compound an average increase of metabolite levels. Out of the 1342 tested compounds (that is Prestwick library + antibiotics), only 35 (less than 3%) induced significant changes in the levels of BCAA intermediates (pvalue ≤ 5e-4 Bonferroni corrected and mean Z-score≥0.5). In addition to chloroxine, clioquinol, CuCl2 and paraquat, we observed that several of the 35 compounds inhibit protein synthesis (for example apramacyn, tobramycin, ribostamacyn…). This is likely reflecting the indirect accumulation of amino acids, including BCAA intermediates, as a results of the inhibition of protein synethesis4,18. H) Growth of wild-type E.coli (blue) and ΔkatG (black) without and with (dashed lines) 7.8 µM of chloroxine. H) Growth of wild-type E.coli (blue), ΔrcsB (black) and rcsB++ (expression of rcsB is under the control of an IPTG-inducible promoter, here we used 1 mM of IPTG) without and with (dashed lines) 7.8 µM of chloroxine. J) Scatter plot of metabolic changes induced by chloroxine vs clioquinol. K) Scatter plot of metabolic changes induced by chloroxine vs paraquat. Some of the largest metabolic changes induced by paraquat involved metabolic intermediates of branched chain amino acids.
Supplementary information
Supplementary Data 1
Supplementary Dataset 1.
Supplementary Data 2
Supplementary Dataset 2.
Supplementary Data 3
Supplementary Dataset 3.
Supplementary Data 4
Supplementary Dataset 4.
Supplementary Data 5
Supplementary Dataset 5.
Supplementary Data 6
Supplementary Dataset 6.
Supplementary Data 7
Supplementary Dataset 7.
Supplementary Data 8
Supplementary Dataset 8.
Source data
Source Data Fig. 1
Data necessary to reproduce all figures.
Source Data Fig. 2
Data necessary to reproduce all figures.
Source Data Fig. 3
Data necessary to reproduce all figures.
Source Data Fig. 4
Data necessary to reproduce all figures.
Source Data Fig. 5
Data necessary to reproduce all figures.
Source Data Fig. 6
Data necessary to reproduce all figures.
Source Data Extended Data Fig. 1
Data necessary to reproduce all figures.
Source Data Extended Data Fig. 2
Data necessary to reproduce all figures.
Source Data Extended Data Fig. 3
Data necessary to reproduce all figures.
Source Data Extended Data Fig. 4
Data necessary to reproduce all figures.
Source Data Extended Data Fig. 5
Data necessary to reproduce all figures.
Source Data Extended Data Fig. 6
Data necessary to reproduce all figures.
Source Data Extended Data Fig. 7
Data necessary to reproduce all figures.
Rights and permissions
About this article
Cite this article
Anglada-Girotto, M., Handschin, G., Ortmayr, K. et al. Combining CRISPRi and metabolomics for functional annotation of compound libraries. Nat Chem Biol 18, 482–491 (2022). https://doi.org/10.1038/s41589-022-00970-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-022-00970-3
This article is cited by
-
Antimicrobial resistance crisis: could artificial intelligence be the solution?
Military Medical Research (2024)
-
Small molecule metabolites: discovery of biomarkers and therapeutic targets
Signal Transduction and Targeted Therapy (2023)
-
Profiling cell envelope-antibiotic interactions reveals vulnerabilities to β-lactams in a multidrug-resistant bacterium
Nature Communications (2023)
-
A high-throughput metabolomics in vitro platform for the characterization of hepatotoxicity
Cell Biology and Toxicology (2023)
-
MIRTH: Metabolite Imputation via Rank-Transformation and Harmonization
Genome Biology (2022)