Abstract
An RNA-involved phase-separation model has been proposed for transcription control. However, the molecular links that connect RNA to the transcription machinery remain missing. Here we find that RNA-binding proteins (RBPs) constitute half of the chromatin proteome in embryonic stem cells (ESCs), some being colocalized with RNA polymerase (Pol) II at promoters and enhancers. Biochemical analyses of representative RBPs show that the paraspeckle protein PSPC1 inhibits the RNA-induced premature release of Pol II, and makes use of RNA as multivalent molecules to enhance the formation of transcription condensates and subsequent phosphorylation and release of Pol II. This synergistic interplay enhances polymerase engagement and activity via the RNA-binding and phase-separation activities of PSPC1. In ESCs, auxin-induced acute degradation of PSPC1 leads to genome-wide defects in Pol II binding and nascent transcription. We propose that promoter-associated RNAs and their binding proteins synergize the phase separation of polymerase condensates to promote active transcription.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the findings of this study are available within the article and in Supplementary information. Sequencing data have been deposited in the GEO database under the accession number GSE150399. Source data are provided with this paper.
References
Roeder, R. G. & Rutter, W. J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature 224, 234–237 (1969).
Cramer, P. Organization and regulation of gene transcription. Nature 573, 45–54 (2019).
Iborra, F. J., Pombo, A., Jackson, D. A. & Cook, P. R. Active RNA polymerases are localized within discrete transcription ‘factories’ in human nuclei. J. Cell Sci. 109, 1427–1436 (1996).
Zobeck, K. L., Buckley, M. S., Zipfel, W. R. & Lis, J. T. Recruitment timing and dynamics of transcription factors at the Hsp70 loci in living cells. Mol. Cell 40, 965–975 (2010).
Cisse, I. I. et al. Real-time dynamics of RNA polymerase II clustering in live human cells. Science 341, 664–667 (2013).
Chong, S. et al. Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science 361, eaar2555 (2018).
Cook, P. R. Predicting three-dimensional genome structure from transcriptional activity. Nat. Genet. 32, 347–352 (2002).
Guenther, M. G., Levine, S. S., Boyer, L. A., Jaenisch, R. & Young, R. A. A chromatin landmark and transcription initiation at most promoters in human cells. Cell 130, 77–88 (2007).
Core, L. J., Waterfall, J. J. & Lis, J. T. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 322, 1845–1848 (2008).
Harlen, K. M. & Churchman, L. S. The code and beyond: transcription regulation by the RNA polymerase II carboxy-terminal domain. Nat. Rev. Mol. Cell Biol. 18, 263–273 (2017).
McCracken, S. et al. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature 385, 357–361 (1997).
Elowitz, M. B., Levine, A. J., Siggia, E. D. & Swain, P. S. Stochastic gene expression in a single cell. Science 297, 1183–1186 (2002).
Darzacq, X. et al. In vivo dynamics of RNA polymerase II transcription. Nat. Struct. Mol. Biol. 14, 796–806 (2007).
Boettiger, A. N. & Levine, M. Synchronous and stochastic patterns of gene activation in the Drosophila embryo. Science 325, 471–473 (2009).
Gebhardt, J. C. et al. Single-molecule imaging of transcription factor binding to DNA in live mammalian cells. Nat. Methods 10, 421–426 (2013).
Rodriguez, J. et al. Intrinsic dynamics of a human gene reveal the basis of expression heterogeneity. Cell 176, 213–226 (2019).
Cho, W. K. et al. RNA Polymerase II cluster dynamics predict mRNA output in living cells. eLife 5, e13617 (2016).
Steurer, B. et al. Live-cell analysis of endogenous GFP-RPB1 uncovers rapid turnover of initiating and promoter-paused RNA Polymerase II. Proc. Natl Acad. Sci. USA 115, E4368–E4376 (2018).
Seila, A. C. et al. Divergent transcription from active promoters. Science 322, 1849–1851 (2008).
Preker, P. et al. RNA exosome depletion reveals transcription upstream of active human promoters. Science 322, 1851–1854 (2008).
Yin, Y. et al. Opposing roles for the lncRNA haunt and its genomic locus in regulating HOXA gene activation during embryonic stem cell differentiation. Cell Stem Cell 16, 504–516 (2015).
Luo, S. et al. Divergent lncRNAs regulate gene expression and lineage differentiation in pluripotent cells. Cell Stem Cell 18, 637–652 (2016).
Li, X. et al. GRID-seq reveals the global RNA-chromatin interactome. Nat. Biotechnol. 35, 940–950 (2017).
Liu, L. C. et al. Insight into novel RNA-binding activities via large-scale analysis of lncRNA-bound proteome and IDH1-bound transcriptome. Nucleic Acids Res. 47, 2244–2262 (2019).
Yin, Y. F. et al. U1 snRNP regulates chromatin retention of noncoding RNAs. Nature 580, 147–150 (2020).
Skalska, L., Beltran-Nebot, M., Ule, J. & Jenner, R. G. Regulatory feedback from nascent RNA to chromatin and transcription. Nat. Rev. Mol. Cell Biol. 18, 331–337 (2017).
Hnisz, D., Shrinivas, K., Young, R. A., Chakraborty, A. K. & Sharp, P. A. A phase separation model for transcriptional control. Cell 169, 13–23 (2017).
Henninger, J. E. et al. RNA-mediated feedback control of transcriptional condensates. Cell 184, 207–225 (2021).
Maharana, S. et al. RNA buffers the phase separation behavior of prion-like RNA binding proteins. Science 360, 918–921 (2018).
Swinburne, I. A., Meyer, C. A., Liu, X. S., Silver, P. A. & Brodsky, A. S. Genomic localization of RNA binding proteins reveals links between pre-mRNA processing and transcription. Genome Res. 16, 912–921 (2006).
Gerstberger, S., Hafner, M. & Tuschl, T. A census of human RNA-binding proteins. Nat. Rev. Genet. 15, 829–845 (2014).
Schwartz, J. C. et al. FUS binds the CTD of RNA polymerase II and regulates its phosphorylation at Ser2. Genes Dev. 26, 2690–2695 (2012).
Ji, X. et al. SR proteins collaborate with 7SK and promoter-associated nascent RNA to release paused polymerase. Cell 153, 855–868 (2013).
Calo, E. et al. RNA helicase DDX21 coordinates transcription and ribosomal RNA processing. Nature 518, 249–253 (2015).
Zeng, Y. et al. Lin28A binds active promoters and recruits Tet1 to regulate gene expression. Mol. Cell 61, 153–160 (2016).
Nozawa, R. S. et al. SAF-A regulates interphase chromosome structure through oligomerization with chromatin-associated RNAs. Cell 169, 1214–1227 (2017).
Guallar, D. et al. RNA-dependent chromatin targeting of TET2 for endogenous retrovirus control in pluripotent stem cells. Nat. Genet. 50, 443–451 (2018).
Xiao, R. et al. Pervasive chromatin-RNA binding protein interactions enable RNA-based regulation of transcription. Cell 178, 107–121 (2019).
Bi, X. et al. RNA targets ribogenesis factor WDR43 to chromatin for transcription and pluripotency control. Mol. Cell 75, 102–116 (2019).
Lu, J. Y. et al. Genomic repeats categorize genes with distinct functions for orchestrated regulation. Cell Rep. 30, 3296–3311 (2020).
Zhang, H. et al. DEAD-box helicase 18 counteracts PRC2 to safeguard ribosomal DNA in pluripotency regulation. Cell Rep. 30, 81–97 (2020).
Graumann, J. et al. Stable isotope labeling by amino acids in cell culture (SILAC) and proteome quantitation of mouse embryonic stem cells to a depth of 5,111 proteins. Mol. Cell. Proteomics 7, 672–683 (2008).
Ebmeier, C. C. et al. Human TFIIH kinase CDK7 regulates transcription-associated chromatin modifications. Cell Rep. 20, 1173–1186 (2017).
Kato, M. et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149, 753–767 (2012).
Lin, Y., Protter, D. S., Rosen, M. K. & Parker, R. Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol. Cell 60, 208–219 (2015).
Ghosal, S., Das, S. & Chakrabarti, J. Long noncoding RNAs: new players in the molecular mechanism for maintenance and differentiation of pluripotent stem cells. Stem. Cells Dev. 22, 2240–2253 (2013).
Vos, S. M. et al. Structure of activated transcription complex Pol II-DSIF-PAF-SPT6. Nature 560, 607–612 (2018).
Goldman, S. R., Ebright, R. H. & Nickels, B. E. Direct detection of abortive RNA transcripts in vivo. Science 324, 927–928 (2009).
Pai, D. A. et al. RNAs nonspecifically inhibit RNA polymerase II by preventing binding to the DNA template. RNA 20, 644–655 (2014).
Ghetti, A., Pinol-Roma, S., Michael, W. M., Morandi, C. & Dreyfuss, G. hnRNP I, the polypyrimidine tract-binding protein: distinct nuclear localization and association with hnRNAs. Nucleic Acids Res. 20, 3671–3678 (1992).
Nishimura, K., Fukagawa, T., Takisawa, H., Kakimoto, T. & Kanemaki, M. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat. Methods 6, 917–922 (2009).
Beltran, M. et al. The interaction of PRC2 with RNA or chromatin is mutually antagonistic. Genome Res. 26, 896–907 (2016).
Qin, H. & Wang, Y. Exploring DNA-binding proteins with in vivo chemical cross-linking and mass spectrometry. J. Proteome Res. 8, 1983–1991 (2009).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Christoforou, A. et al. A draft map of the mouse pluripotent stem cell spatial proteome. Nat. Commun. 7, 8992 (2016).
Meshorer, E. et al. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev. Cell 10, 105–116 (2006).
Lee, C. H., Wu, J. & Li, B. Chromatin remodelers fine-tune H3K36me-directed deacetylation of neighbor nucleosomes by Rpd3S. Mol. Cell 52, 255–263 (2013).
Li, B. et al. Combined action of PHD and chromo domains directs the Rpd3S HDAC to transcribed chromatin. Science 316, 1050–1054 (2007).
Bi, X., Zhang, X. & Shen, X. Transcriptome-wide profiling of protein-RNA interactions by cross-linking and immunoprecipitation mediated by FLAG-biotin tandem purification. J. Vis. Exp. https://doi.org/10.3791/60730 (2020).
Eisen, T. J., Eichhorn, S. W., Subtelny, A. O. & Bartel, D. P. MicroRNAs cause accelerated decay of short-tailed target mRNAs. Mol. Cell 77, 775–785 (2020).
Acknowledgements
We thank the members of the Shen Laboratory for insightful discussions. This work was supported in part by the National Basic Research Program of China (nos. 2018YFA0107604 and 2017YFA0504204 to X.S.); the National Key R&D Program of China (no. 2018YFC1004500 to B.L.); the National Natural Science Foundation of China (nos. 31925015 and 31829003 to X.S. and 32030019 and 31872817 to B.L.); and Beijing Advanced Innovation Center for Structural Biology at Tsinghua University (to X.S.).
Author information
Authors and Affiliations
Contributions
X.S. supervised the study. X.S. and W.S. conceived of and designed the experiments. W.S. performed the majority of experiments, with help from X.B., B.G., Z.L. and W.R., and conducted bioinformatics analysis with assistance from Y.X., X.B. and J.Y.L. X.B. performed ChIP–seq of FB(EXO)PSPC1, UTP3, UTP6 and CIRH1A and CLIP–seq of PSPC1. The in vitro transcription system was designed and set up by B.L. and J.W. W.S. and Y.P. performed in vitro transcription assays with the help of M.P. Y.Y. performed ChIP–seq of SAFB1, SAFB2 and hnRNPU. W.Z., X.J. and H.D. provided technical assistance/suggestions for MS analysis. Z.W., K.W., G.Z., T.L. and J.W. contributed with assistance/suggestions for experiments. X.H. performed ESC total proteome analysis. X.S. and W.S. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemical Biology thanks Huasong Lu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Abundant associations of RBPs with chromatin in ESCs.
a, Schemes showing quantitative analysis of chromatin proteomes under various treatments by three mass spec (MS) methods. (i) Label-free MS quantification (LBQ); (ii) Tandem mass tag (TMT); (iii) Stable isotope labeling with amino acids (SILAC). Transcription (TXN) inhibition: ActD (actinomycin D, 1 μg/ml) or TPL (triptolide, 1 μM). RNase: RNase A (1 mg/ml). DMSO/Mock: mock treatment for transcription inhibition or RNase treatment. b, Overlap between two biological replicates of the chromatin proteome. P < 2.2e-16, two-sided Fisher’s exact test. c, Correlation analysis of two biological replicates of the chromatin proteome. The x-axis and y-axis represent the abundance of each protein identified in the two replicates, indicated by -log10 (iBaq ratio) (Methods; Supplementary Table 1). d, Gene ontology (GO) analysis of chromatin proteins (n = 1,357). The total proteome from ESCs (n = 2,854) was used as background. Selected GO terms (p < 1.0e-10) are shown on the y-axis. The x-axis shows enrichment significance by -log10 (p-value). Red bars represent terms related to RNA processes; black bars indicate terms associated with transcription and chromatin functions. For each GO term, the number of functionally associated genes identified from analysis of the chromatin proteome and the total number of functionally associated genes expressed in ESCs are indicated sequentially. The numbers in the brackets indicate the fold enrichment. P-values, two-sided Fisher-exact tests performed by DAVID. e, Comparison of the chromatin proteome (n = 1,357) with the RBP repertoire (n = 1,542) and the ESC total proteome (n = 2,854). The numbers of chrRBPs (red, 512) and non-chrRBPs (black, 380) are indicated in bold. See also Supplementary Table 2. f, Biochemical characterization of chrRBPs and non-chrRBPs. (i) Density distribution curve of the isoelectric points of chrRBPs and non-chrRBPs. The blue arrow indicates a shift in the distribution of isoelectric point. (ii) Cumulative distribution curve showing the content of intrinsically disordered regions (IDR) in chrRBPs or non-chrRBPs. P-values, two-sided Kolmogorov-Smirnov test. See also Supplementary Table 2.
Extended Data Fig. 2 Dynamic chromatin associations of RBPs mediated by transcription and RNA.
a, Heatmap showing the average fold-change (FC) score of chromatin abundance for representative proteins including hnRNPs, spliceosome proteins and unchanged proteins. Numerous hnRNPs and splicing factors are dependent on both RNA and transcription for their chromatin binding. In comparison, the chromatin-binding activities of transcription factors (such as UTF1 and ESRRB) and epigenetic enzymes (such as DNMT1, EZH2, WDR5, topoisomerases, and DNA helicases) were less likely to be affected. The ratio calculation is described in Methods. Data are shown as the mean of 4 biological replicates for ActD and RNase, and 3 replicates for TPL. See also Supplementary Table 3. b, GO analysis of chromatin proteins that are insensitive to transcription inhibition and RNase treatment (n = 401). The ESC chromatin proteome was used as the background. The x-axis shows enrichment significance by -log10 (p-value). The top enriched terms are shown on the y-axis. For each GO term, the number of functionally associated genes identified from the input list and the background list are indicated sequentially. The numbers in the brackets indicate the enrichment fold. P-values, two-sided Fisher-exact tests performed by DAVID. c, Summary of the effects of transcription (TXN) and RNA on chromatin-RBP associations. See also Supplementary Table 3. d, Chromatin fraction and western-blot analysis of selected chrRBPs upon treatments with transcription inhibitors or RNase A. The corresponding FC score in the mass spec data is shown in heatmap (right). ActD: low (10 ng/ml) or high (1 μg/ml). RBPs are classified into 3 groups based on their sensitivity to inhibition of Pol I or Pol II transcription (TXN) or RNA. Because nascent transcripts are loaded and protected by a battery of RBPs once they emerge from Pol II, we cannot rule out incomplete degradation of RNA by treatment with RNase A. Thus, despite an overall decrease of chrRBP associations with chromatin, the role of RNA in recruiting and mediating chrRBPs to chromatin could still be underestimated based on the observed effects of RNase treatment. Experiment was repeated twice with similar results.
Extended Data Fig. 3 chrRBPs tend to interact with Pol II and modulate transcription.
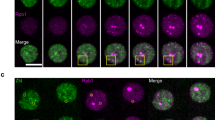
a, Fluorescence activated cell sorting of EU incorporation after RBP knockdown for 60 hours. The x-axis shows EU intensity. The y-axis shows cell numbers normalized to max (%). Two biological replicates are shown for controls (grey) and RBP knockdown (red). b, RT-qPCR analysis of the relative expression of RBPs upon knockdown for 60 hours. Data are shown as mean of 2 biological replicates with different shRNAs. c, Co-immunoprecipitation (co-IP) and western blot analysis of endogenous proteins. Pol II in different phosphorylation states captured all 8 tested chrRBPs. Capture of various phosphorylation states of Pol II was confirmed by western blots using the corresponding antibody in each IP (bottom). Benzonase was added during cell lysis and co-IP. 8WG16 and Ser5P, hypo-phosphorylated (hypoP) and serine 5-phosphorylated Pol II that represent initiating Pol II. Ser2P, serine 2-phosphorylated Pol II that represents elongating Pol II. d, Reciprocal co-IP of various chrRBPs captured Pol II. Biotin-mediated affinity purification (bio-AP) was performed (+ benzonase) in ESCs that stably express individual FLAG-biotin tagged chrRBPs. ESCs expressing FLAG-biotin tagged GFP were used as the negative control. Of note, flavopiridol can reduce elongating transcripts by inhibiting Pol II pause-release, but results in higher promoter-associated transcripts39. Blue arrows indicate exogenously expressed FLAG-tagged proteins. e, FLAG-mediated co-immunoprecipitation (FLAG-IP) of AID-FB(KI)PSPC1 ESCs and western-blot analysis. ESCs treated with IAA for 24 hours were used as a negative control. Benzonase was present during cell lysis and FLAG-IP procedures. RNase A was added during washes. f, Co-immunostaining analysis of PSPC1 with pan-Pol II. The experiment was performed in AID-FB(KI)PSPC1 cells and anti-FLAG antibody was used to image endogenously tagged PSPC1 proteins. g, Co-immunostaining analysis of PSPC1 with TBP. This experiment was performed in wild-type ESCs and PSPC1 antibody was used to detect endogenous proteins. h, FRAP analysis showing the fast recovery of mCherry-PSPC1 puncta. Related to Fig. 1i. i, Immunofluorescence analysis of PSPC1 upon treatment with 10% 1,6-hexanediol for 2 minutes. Mock: PBS. Anti-PSPC1 antibody was used for imaging. For panels e-i, experiments were repeated twice with similar results.
Extended Data Fig. 4 PSPC1 promotes the incorporation of unphosphorylated CTD into TBP condensates.
a, Analysis of IDR and LCS in PSPC1 and TBP. The PONDR score indicates the probability of a region being disordered. The distributions of representative amino acids are shown at the bottom. The regions in yellow indicate the disordered regions which contain the hydrophobic G/P-rich or Q-rich sequences respectively (arrow). b, Coomassie blue staining of purified proteins. The blue arrows indicate the main band of the corresponding protein. c, In vitro droplet formation of recombinant proteins. d-e, Phase diagram of PSPC1-CTD (d) or TBP-CTD (e) droplets in the presence of different concentrations of total RNA isolated from ESCs. We used 0.6 μM of CTD in all assays. f-g, Droplet formation assays of TBP (5 μM) and CTD (0.6 μM) with full-length (FL) or mutant PSPC1 (5 μM) or mCherry (5 μM) in the presence of 10% dextran. Representative pictures and quantification are shown in panel f and g respectively. In panel g, the y-axis is log2 (relative droplet size). The whiskers are drawn within 10-90 percentile. Points below and above the whiskers are not shown. The median droplet size is 0.71 μm2 for mCherry (n = 2,641), 1.50 μm2 for PSPC1FL (n = 2,932), 0.99 μm2 for PSPC1ΔLCS (n = 2,097), and 1.42 μm2 for PSPC1RRMmut (n = 4,758). P-values, two-sided Student’s t-test, are shown as indicated. h, Fusion of TBP-CTD-PSPC1 droplets. i, Phase-separated droplets composed of TBP, CTD and PSPC1 with or without 10% 1,6-hexanediol. Ctrl: mock treatment. j-k, Droplets sedimentation and western-blot assays. The schematic diagram and quantification are shown in panel j and k, respectively. Representative western-blot result is shown in Fig. 2b. The pellet fraction ratio P/(S + P) was shown as mean ± s.d. P-values, two-sided student’s t-tests, are shown as indicated. N = 2 independent experiments for ‘mCherry’ and ‘PSPC1RRMmut’, N = 3 for ‘PSPC1FL’ and ‘PSPC1ΔLCS2’. l, In vitro droplet formation of recombinant PSPC1RRMmut and PSPC1ΔLCS2 proteins. For panels b-e, g-h, experiments were repeated twice with similar results. Dextran was used as indicated.
Extended Data Fig. 5 PSPC1 promotes CTD phosphorylation and release.
a, Kinase assays with CDK9 (left) and CDK7 (right). Quantification of the ratio of phosphorylated CTD versus total CTD is shown at the bottom. Additional replicate was shown in Fig. 2c. b-c, Time-lapse imaging analysis of CTD release. Panel b shows the relative release rate of CTD (y-axis) (Methods). Data are shown as mean ± s.d of droplets. P-value, two-tailed Student’s t-test for the comparison of max rate between the two groups. Panel c shows representative images. Related to Fig. 2d. d, Time-lapse sedimentation analysis of released CTD. The samples are the same with Fig. 2e. e, RNA promotes PSPC1 phase separation by droplets sedimentation. Quantification is shown at the bottom of each panel. f-g, Quantifications of total fluorescence intensity of TBP (f) and droplet size (g) for data shown in Fig. 2f. P-values, two-sided Student’s t-test (n.s., not significant). f, Data are shown as mean ± s.d. of 10 fields. P-values are shown in the sequence of ‘no RNA’, ‘25’, ‘50’, and ‘100’. ‘mCherry’ vs ‘PSPC1FL’: 2.28e-7, 7.75e-14, 3.43e-11, and 5.89e-17. ‘PSPC1FL’ vs ‘PSPC1RRMmut’: 0.2122, 2.43e-6, 2.59e-4 and 1.66e-7. ‘PSPC1FL’ vs ‘PSPC1ΔLCS2’: 0.0379, 5.77e-6, 4.89e-7 and 1.26e-10. g, Droplet sizes are presented as log2 (relative droplet size). The whiskers are drawn within 10-90 percentile. Points beyond the whiskers are not shown. The median droplet sizes and p-values are shown in the sequence of ‘no RNA’, ‘25’, ‘50’, and ‘100’. mCherry: 0.71 (n = 2,641), 0.56 (n = 2,555), 0.40 (n = 1,698), and 0.40 (n = 1,961). PSPC1FL: 1.50 (n = 2,932), 2.19 (n = 2,732), 3.69 (n = 1,838), and 2.79 (n = 3,663). PSPC1RRMmut: 1.42 (n = 4,758), 1.37 (n = 5,029), 1.50 (n = 7,073), and 1.50 (n = 8,567). PSPC1ΔLCS2: 0.99 (n = 2,097), 0.94 (n = 1,313), 0.82 (n = 1,436), and 1.03 (n = 1,429). P-values: ‘mCherry’ vs ‘PSPC1FL’: 5.60e-241, < 5.60e-241, 3.00e-236 and 1.65e-143. ‘PSPC1FL’ vs ‘PSPC1RRMmut’: 3.41e-49, 6.88e-137, 1.20e-231, and 3.56e-128. ‘PSPC1FL’ vs ‘PSPC1ΔLCS2’: 8.86e-85, 3.0e-101, 1.18e-170 and 5.48e-70. Dextran (10%) was used in panels b-d and f-g.
Extended Data Fig. 6 RNA synergizes with PSPC1 in promoting CTD incorporation, phosphorylation and release.
a-b, Effects of RNA on CTD release by time-lapse imaging analysis. Panel a shows images taken of representative droplets. Related to Fig. 2i. This assay included 10% dextran. Panel b shows the quantification of CTD release rate (Methods). Data are shown as mean ± s.d. of 113 droplets for the ‘PSPC1FL, no RNA’ group and 69 droplets for the ‘PSPC1FL, + RNA’ group. P-value, two-tailed Student’s t-test for the comparison of max rate between the two groups. c, Schematic diagram showing the interplay of PSPC1 and RNA in promoting CTD incorporation and subsequent phosphorylation and release. TBP alone has weak ability to phase separate and trap CTD within its droplets in the presence of dextran (i). Addition of PSPC1 enhances this phase separation and produces larger droplets that concentrate more CTD inside (ii). RNA further synergizes with PSPC1 to drastically promote phase separation and CTD incorporation (iii). By contrast, in the absence of PSPC1, RNA evicts CTD from TBP droplets (iv). Upon activation by CTD kinases (v), efficient compartmentalization and concentration of CTD inside TBP-PSPC1-RNA droplets lead to stronger phosphorylation and faster release of CTD compared to TBP and TBP-PSPC1 droplets. Note that RNA synergizes with PSPC1 in a manner that critically depends on the phase-separation and RNA-binding activities of PSPC1. Thus, in vitro assays with defined components allow us to biochemically dissect the more complex processes of Pol II engagement and release in cells.
Extended Data Fig. 7 PSPC1 promotes Pol II transcription in vitro.
a, Schematic diagram of the template DNA. ‘BR’ stands for bubble right (the 601 sequence). The template was labeled with either biotin (panel c) or Cy5.5 (panels d-f). b, Silver staining of purified Pol II. c, Schematic diagram (upper) and autoradiography (lower) of in vitro transcription with various DNA templates and nucleotides as indicated. d, Autoradiography of in vitro transcription with the template in the presence of NTPs and 32P-CTP. Additional replicate was shown in Fig. 3b. Besides the full-length (~278 nt) transcript, we detected high levels of abortive short transcripts (15-25 nt), which is consistent with published results48. It may result from the frequent fall-off of Pol II from the template due to dsDNA torsion during transcription. The asterisk indicates spike-in control (~170 nt). The relative intensities of full-length/short transcripts normalized to spike-in control were quantified and indicated at the bottom. e, Titration of heparin to reduce loosely docked Pol II on the template by ESMA. The concentration of heparin used in lanes 3-4 was chosen for in vitro transcription and EMSA assays. f, Titration of a competitor DNA to reduce nonspecific binding of PSPC1 to the BR template. The bottom panel shows quantification of the unbound Cy5.5-labeled BR template (indicated as ‘DNA’ by an arrowhead). In the absence of a competitor DNA (lanes 2-3 vs lane 1), the addition of PSPC1FL led to decreased signals of unbound templates in the bottom and increased BR signals (likely bound by PSPC1) that were stuck in the well on the top. When a 100:1 ratio of the competitor to BR template was used (lanes 8-9), the binding of PSPC1 to the BR template was minimized, while Pol II binding was not affected. We thus used the 100:1 ratio for in vitro transcription and EMSA assays.
Extended Data Fig. 8 PSPC1 stabilizes Pol II during in vitro transcription.
a, Effects of PSPC1FL on the binding of Pol II to BR template during in vitro transcription in the absence or presence of heparin by ESMA. The free template (‘DNA’) and the supershifted ‘Pol II:DNA’ bands are indicated on the left. The bands marked by single asterisk is likely to be a non-specified byproduct during BR template assembly and gel purification. The bands marked by double asterisks are likely to be a R-loop, given its sensitivity to RNase H. Heparin effectively removed the docking Pol II from the template in the absence of NTPs (comparing lane 9 to lane 3), but had negligible effects on the stalled or elongating Pol II (comparing lane 10 to 4 and lane 11 to 5). Importantly, addition of recombinant PSPC1FL consistently enhanced the Pol II:DNA signals in both the absence (lanes 6-8 vs 3-5) and the presence of heparin (lanes 12-14 vs 9-11). PSPC1FL did not form a stable complex with Pol II, suggesting weak interactions. b-c, Effects of PSPC1 mutants on the binding of Pol II to BR template during in vitro transcription (heparin included). PSPC1RRMmut and PSPC1ΔLCS2 had negligible effects on Pol II binding (panel b, lanes 8-11). Representative results of independent experiments are shown in panels b and c. d, Effects of recombinant proteins on the binding of Pol II to the BR template during in vitro transcription by ESMA. For panels b and d, the relative intensity of supershifted Pol II:DNA signals was indicated at the bottom. e, Phase diagram of PTBP1-CTD droplets in the presence of ESC RNA. We used 0.6 μM of CTD in all assays in the presence of 10% dextran. PTBP1 poorly phase separated in the absence of dextran. For panel a, d and e, experiments were repeated twice with similar results.
Extended Data Fig. 9 PSPC1 co-localizes with Pol II and its acute degradation impairs transcription.
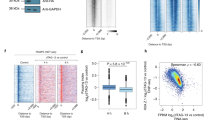
a-e, ChIP-seq analysis of PSPC1. Panel a shows overlap between targets identified by two biological replicates of AID-FB(KI)PSPC1 ChIP-seq and one ectopically tagged PSPC1 (FB(EXO)PSPC1) ChIP-seq. Panels b-d show the overlap of AID-FB(KI)PSPC1-targeted promoters (n = 5,262, overlapping promoters of two-biological replicates) and Pol II (hypo/Ser5P)-targeted promoters (13,791) (b), hypoP (c) or Ser5P ChIP-seq (d) respectively. P-values by two-sided Fisher’s exact test are shown as indicated. Panel e shows UCSC genome browser view of ChIP-seq, CLIP-seq, TT-seq and EU-seq at representative loci. f, Average read density of PSPC1 CLIP-seq of two biological replicates on PSPC1 ChIP-seq summits (i) and vice versa (ii). g, Anti-FLAG ChIP-qPCR of PSPC1 and GFP proteins that were transiently expressed in ESCs. Data are shown as mean ± s.d. of 3 biological replicates. h, Effect of 1,6-hexanediol on AID-FB(KI)PSPC1 binding to its targets. Data are shown as mean of 2 independent biological replicates. i, Anti-FLAG immunofluorescence analysis. Left, representative images; right, quantification of relative fluorescence intensities along the yellow lines. j, Time-course ChIP-qPCR analysis of Pol II Ser5P (left) and Ser2P (right) upon PSPC1 degradation. Data are shown as mean ± s.d. of biological replicates. Ser5P ChIP: 2 replicates; Ser2P ChIP: 4 replicates for ‘no IAA’ and ‘+ IAA 6 h’; 3 replicates for ‘+ IAA 3 h’ and ‘+ IAA 24 h’. P-values by one-sided Student’s t-test are shown as indicated. For panels i, and j, experiments were repeated twice with similar results. For panels g and h, the relative fold of enrichment at each target was normalized to OLFR1437. P-values by one-sided (h and j) or two-sided (g) Student’s t-test are shown as indicated.
Extended Data Fig. 10 Genome-wide co-occupancy of chrRBPs with Pol II at promoters and enhancers.
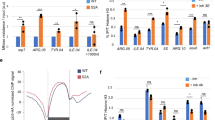
a, Metagene analysis of ChIP-seq signals of various RBPs across all mouse genes (n = 32,944) (i) and enhancers (ii). The y-axis is reads per million reads (RPM). Related to Fig. 6a. b, Heatmap showing hierarchical clustering of ChIP-seq signals of various RBPs, Pol II, transcription and chromatin regulators, and histone marks in ESCs. The color indicates the Pearson correlation value. c-d, Phase-separation assay of various RBPs with the CTD. The concentrations of GFP-tagged CTD, mCherry, and mCherry-tagged PSPC1, PTBP1 and LCS domain of hnRNPL (hnRNPLLCS) used here are 0.6 μM, 45 μM, 5 μM, 10 μM and 20 μM respectively. Representative pictures are shown in panel c. Quantification for RBP-CTD droplet size (left) and the total CTD fluorescence intensity (right) in the droplet are shown in panel d. In the left panel, the y-axis is log2 (relative droplet size). The whiskers are drawn within 10-90 percentile. Points below and above the whiskers are not shown. The median droplet size is 1.04 μm2 for PSPC1 (n = 782), 0.37 μm2 for hnRNPLLCS (n = 6,158), 0.48 μm2 for PTBP1 (n = 2,374), and 9.60 μm2 for all 3 RBPs together (n = 554). In the right panel, the y-axis shows the sum of fluorescence intensity of CTD in droplets in each field of view. Data are shown as mean ± s.d. of 3 fields for ‘PSPC1’, ‘hnRNPLLCS’ and ‘PTBP1’, and 5 fields for ‘3RBPs’. e, Promoter tethering assay in 293T cells. The y-axis shows the relative luciferase intensity normalized to the Gal4DBD control. Data are shown as mean ± s.d. For panels d and e, p-values by two-sided Student’s t-test are shown as indicated.
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Supplementary Tables
Supplementary Table 1: Abundance analysis of the ESC chromatin proteome; Supplementary Table 2: Biochemical features of chrRBPs versus non-chrRBPs; Supplementary Table 3: Summary of effects of transcription inhibitors and RNA degradation on the chromatin proteome; Supplementary Table 4: Summary of PSPC1 ChIP–seq targets. Supplementary Table 5: Summary of ChIP–seq binding of various RBPs at promoters and enhancers; Supplementary Table 6: Lists of primers, adapters and reagents in this study.
Supplementary Video 1
PSPC1 promotes CTD release from TBP droplets.
Supplementary Video 2
RNA promotes CTD release from TBP-PSPC1 droplets.
Supplementary Video 3
CTD release from TBP, TBP-PSPC1 and TBP-PSPC1-RNA droplets is ATP dependent.
Source data
Source Data Fig. 1
Unprocessed immunoblots.
Source Data Fig. 2
Unprocessed immunoblots.
Source Data Fig. 3
Unprocessed gels.
Source Data Fig. 4
Unprocessed immunoblots.
Source Data Fig. 5
Unprocessed immunoblots.
Source Data Extended Data Fig. 2
Unprocessed immunoblots.
Source Data Extended Data Fig. 3
Unprocessed immunoblots.
Source Data Extended Data Fig. 4
Unprocessed gels.
Source Data Extended Data Fig. 5
Unprocessed immunoblots.
Source Data Extended Data Fig. 7
Unprocessed gels.
Source Data Extended Data Fig. 8
Unprocessed gels.
Rights and permissions
About this article
Cite this article
Shao, W., Bi, X., Pan, Y. et al. Phase separation of RNA-binding protein promotes polymerase binding and transcription. Nat Chem Biol 18, 70–80 (2022). https://doi.org/10.1038/s41589-021-00904-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-021-00904-5
This article is cited by
-
RBM22 regulates RNA polymerase II 5′ pausing, elongation rate, and termination by coordinating 7SK-P-TEFb complex and SPT5
Genome Biology (2024)
-
Chromatin balances cell redox and energy homeostasis
Epigenetics & Chromatin (2023)
-
Phase separation of protein mixtures is driven by the interplay of homotypic and heterotypic interactions
Nature Communications (2023)
-
Homodimer-mediated phosphorylation of C/EBPα-p42 S16 modulates acute myeloid leukaemia differentiation through liquid-liquid phase separation
Nature Communications (2023)
-
Pharmacological perturbation of the phase-separating protein SMNDC1
Nature Communications (2023)