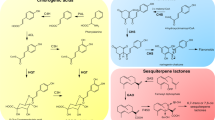
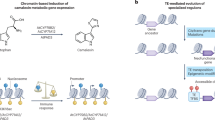
Abstract
As sessile organisms, plants evolved elaborate metabolic systems that produce a plethora of specialized metabolites as a means to survive challenging terrestrial environments. Decades of research have revealed the genetic and biochemical basis for a multitude of plant specialized metabolic pathways. Nevertheless, knowledge is still limited concerning the selective advantages provided by individual and collective specialized metabolites to the reproductive success of diverse host plants. Here we review the biological functions conferred by various classes of plant specialized metabolites in the context of the interaction of plants with their surrounding environment. To achieve optimal multifunctionality of diverse specialized metabolic processes, plants use various adaptive mechanisms at subcellular, cellular, tissue, organ and interspecies levels. Understanding these mechanisms and the evolutionary trajectories underlying their occurrence in nature will ultimately enable efficient bioengineering of desirable metabolic traits in chassis organisms.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kenrick, P. & Crane, P. R. The origin and early evolution of plants on land. Nature 389, 33–39 (1997).
Li, F.-S. & Weng, J.-K. Demystifying traditional herbal medicine with modern approach. Nat. Plants 3, 17109 (2017).
De Smet, P. A. The role of plant-derived drugs and herbal medicines in healthcare. Drugs 54, 801–840 (1997).
Lau, W. & Sattely, E. S. Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 349, 1224–1228 (2015). Leveraging coexpression analysis and transient transformation of N. benthamiana, the authors identify the biosynthetic pathway of the etoposide aglycone podophyllotoxin in mayapple.
Lichman, B. R. et al. The evolutionary origins of the cat attractant nepetalactone in catnip. Sci. Adv. 6, eaba0721 (2020). Using a comparative phylogenomics approach, the authors delineate the process underlying the reemergence of iridoid biosynthesis in catnip in the Nepeta lineage, which involves the assembly of a nepetalactone biosynthetic gene cluster.
Kessler, A. & Kalske, A. Plant secondary metabolite diversity and species interactions. Annu. Rev. Ecol. Evol. Syst. 49, 115–138 (2018).
Bruce, T. J. A. & Pickett, J. A. Perception of plant volatile blends by herbivorous insects—finding the right mix. Phytochemistry 72, 1605–1611 (2011).
Kessler, D. et al. How scent and nectar influence floral antagonists and mutualists. eLife 4, e07641 (2015).
Erb, M. & Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 70, 527–557 (2019).
Mhlongo, M. I., Piater, L. A., Madala, N. E., Labuschagne, N. & Dubery, I. A. The chemistry of plant–microbe interactions in the rhizosphere and the potential for metabolomics to reveal signaling related to defense priming and induced systemic resistance. Front. Plant Sci. 9, 112 (2018).
Schandry, N. & Becker, C. Allelopathic plants: models for studying plant-interkingdom interactions. Trends Plant Sci. 25, 176–185 (2020).
Weston, L. A. & Mathesius, U. Flavonoids: their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol. 39, 283–297 (2013).
Voges, M. J. E. E. E., Bai, Y., Schulze-Lefert, P. & Sattely, E. S. Plant-derived coumarins shape the composition of an Arabidopsis synthetic root microbiome. Proc. Natl Acad. Sci. USA 116, 12558–12565 (2019).
Korenblum, E. et al. Rhizosphere microbiome mediates systemic root metabolite exudation by root-to-root signaling. Proc. Natl Acad. Sci. USA 117, 3874–3883 (2020). This work demonstrates that the tomato rhizosphere microbiome affects the chemical composition of root exudation through a systemic root–root signaling mechanism dubbed systemically induced root exudation of metabolites (SIREM).
Manohar, M. et al. Plant metabolism of nematode pheromones mediates plant–nematode interactions. Nat. Commun. 11, 208 (2020).
Hu, L. et al. Root exudate metabolites drive plant–soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 9, 2738 (2018).
Falik, O., Hoffmann, I. & Novoplansky, A. Say it with flowers: flowering acceleration by root communication. Plant Signal. Behav. 9, e28258 (2014).
Huang, W., Zwimpfer, E., Hervé, M. R., Bont, Z. & Erb, M. Neighbourhood effects determine plant–herbivore interactions below-ground. J. Ecol. 106, 347–356 (2018).
Rasmann, S. et al. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434, 732–737 (2005).
Huang, W., Gfeller, V. & Erb, M. Root volatiles in plant–plant interactions. II. Root volatiles alter root chemistry and plant–herbivore interactions of neighbouring plants. Plant Cell Environ. 42, 1964–1973 (2019).
Gfeller, V. et al. Root volatiles in plant–plant interactions. I. High root sesquiterpene release is associated with increased germination and growth of plant neighbours. Plant Cell Environ. 42, 1950–1963 (2019).
Rand, K. et al. Differences in monoterpene biosynthesis and accumulation in Pistacia palaestina leaves and aphid-induced galls. J. Chem. Ecol. 43, 143–152 (2017).
Zhang, P.-J. et al. Airborne host–plant manipulation by whiteflies via an inducible blend of plant volatiles. Proc. Natl Acad. Sci. USA 116, 7387–7396 (2019).
Wang, X. et al. A novel Meloidogyne incognita chorismate mutase effector suppresses plant immunity by manipulating the salicylic acid pathway and functions mainly during the early stages of nematode parasitism. Plant Pathol. 67, 1436–1448 (2018).
Lanver, D. et al. Ustilago maydis effectors and their impact on virulence. Nat. Rev. Microbiol. 15, 409–421 (2017).
Schuurink, R. & Tissier, A. Glandular trichomes: micro‐organs with model status? N. Phytol. 225, 2251–2266 (2020).
Ramos, M. V., Demarco, D., da Costa Souza, I. C. & de Freitas, C. D. T. Laticifers, latex, and their role in plant defense. Trends Plant Sci. 24, 553–567 (2019).
Zhang, H., Wang, L., Deroles, S., Bennett, R. & Davies, K. New insight into the structures and formation of anthocyanic vacuolar inclusions in flower petals. BMC Plant Biol. 6, 29 (2006).
Ngan, N. T. T. et al. Cytotoxic phenanthrenes and phenolic constituents from the tubers of Dioscorea persimilis. Phytochem. Lett. 40, 139–143 (2020).
Levin, D. A. The role of trichomes in plant defense. Q. Rev. Biol. 48, 3–15 (1973).
Livingston, S. J. et al. Cannabis glandular trichomes alter morphology and metabolite content during flower maturation. Plant J. 101, 37–56 (2020).
McDowell, E. T. et al. Comparative functional genomic analysis of Solanum glandular trichome types. Plant Physiol. 155, 524–539 (2011).
Leong, B. J. et al. Evolution of metabolic novelty: a trichome-expressed invertase creates specialized metabolic diversity in wild tomato. Sci. Adv. 5, eaaw3754 (2019).
Weinhold, A. & Baldwin, I. T. Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. Proc. Natl Acad. Sci. USA 108, 7855–7859 (2011).
Hagel, J. M., Yeung, E. C. & Facchini, P. J. Got milk? The secret life of laticifers. Trends Plant Sci. 13, 631–639 (2008).
Bauer, G. et al. Investigating the rheological properties of native plant latex. J. R. Soc. Interface 11, 20130847 (2014).
Singh, A., Menéndez-Perdomo, I. M. & Facchini, P. J. Benzylisoquinoline alkaloid biosynthesis in opium poppy: an update. Phytochem. Rev. 18, 1457–1482 (2019).
Huber, M. et al. A latex metabolite benefits plant fitness under root herbivore attack. PLoS Biol. 14, e1002332 (2016).
Abarca, L. F. S., Klinkhamer, P. G. L. & Choi, Y. H. Plant latex, from ecological interests to bioactive chemical resources. Planta Med. 85, 856–868 (2019).
Freitas, C. D. T. et al. Identification, characterization, and antifungal activity of cysteine peptidases from Calotropis procera latex. Phytochemistry 169, 112163 (2020).
Konno, K. Plant latex and other exudates as plant defense systems: roles of various defense chemicals and proteins contained therein. Phytochemistry 72, 1510–1530 (2011).
Bernays, E. A., Singer, M. S. & Rodrigues, D. Trenching behavior by caterpillars of the Euphorbia specialist, Pygarctia roseicapitis: a field study. J. Insect Behav. 17, 41–52 (2004).
Erb, M. & Kliebenstein, D. J. Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiol. 184, 39–52 (2020).
Lynch, J. H. et al. Modulation of auxin formation by the cytosolic phenylalanine biosynthetic pathway. Nat. Chem. Biol. 16, 850–856 (2020).
Kim, J. I., Zhang, X., Pascuzzi, P. E., Liu, C. & Chapple, C. Glucosinolate and phenylpropanoid biosynthesis are linked by proteasome-dependent degradation of PAL. N. Phytol. 225, 154–168 (2020). Analysis of the impact of indole glucosinolate intermediates on flux toward production of phenylpropanoids reveals the intertwined roles of metabolism, transcriptional control and protein turnover on co-regulation of distinct metabolic pathways.
Katz, E. et al. The glucosinolate breakdown product indole-3-carbinol acts as an auxin antagonist in roots of Arabidopsis thaliana. Plant J. 82, 547–555 (2015).
Katz, E. & Chamovitz, D. A. Wounding of Arabidopsis leaves induces indole-3-carbinol-dependent autophagy in roots of Arabidopsis thaliana. Plant J. 91, 779–787 (2017).
Malinovsky, F. G. et al. An evolutionarily young defense metabolite influences the root growth of plants via the ancient TOR signaling pathway. eLife 6, e29353 (2017). Glucosinolates are specialized defense compounds produced by Brassicaceae plants against herbivores. This work identifies one of these glucosinolates, 3-hydroxypropylglucosinolate, that regulates root growth through influencing the TOR complex.
Weng, J.-K., Ye, M., Li, B. & Noel, J. P. Co-evolution of hormone metabolism and signaling networks expands plant adaptive plasticity. Cell 166, 881–893 (2016).
Jia, K.-P. et al. Anchorene is a carotenoid-derived regulatory metabolite required for anchor root formation in Arabidopsis. Sci. Adv. 5, eaaw6787 (2019). A carotenoid-derived dialdehyde (diapocarotenoid) is identified as the specific signal needed for anchor root formation in Arabidopsis.
Ablazov, A. et al. The apocarotenoid zaxinone is a positive regulator of strigolactone and abscisic acid biosynthesis in Arabidopsis roots. Front. Plant Sci. 11, 578 (2020).
Dickinson, A. J. et al. A plant lipocalin is required for retinal-mediated de novo root organogenesis. Preprint at bioRxiv https://doi.org/10.1101/2020.11.09.375444 (2020).
Boachon, B. et al. Natural fumigation as a mechanism for volatile transport between flower organs. Nat. Chem. Biol. 15, 583–588 (2019). This work reveals the hormone-like function of terpenoids and their aerial transport within enclosed spaces of plant tissues, which impacts organ development and reproductive fitness.
Li, J. et al. The decoration of specialized metabolites influences stylar development. eLife 7, e38611 (2018).
Alam, M. T. et al. The self-inhibitory nature of metabolic networks and its alleviation through compartmentalization. Nat. Commun. 8, 16018 (2017).
Knudsen, C., Gallage, N. J., Hansen, C. C., Møller, B. L. & Laursen, T. Dynamic metabolic solutions to the sessile life style of plants. Nat. Prod. Rep. 35, 1140–1155 (2018).
Schenck, C. A. & Last, R. L. Location, location! Cellular relocalization primes specialized metabolic diversification. FEBS J. 287, 1359–1368 (2020).
Widhalm, J. R. et al. Identification of a plastidial phenylalanine exporter that influences flux distribution through the phenylalanine biosynthetic network. Nat. Commun. 6, 8142 (2015).
Lynch, J. H. et al. Multifaceted plant responses to circumvent Phe hyperaccumulation by downregulation of flux through the shikimate pathway and by vacuolar Phe sequestration. Plant J. 92, 939–950 (2017).
Shitan, N. & Yazaki, K. Dynamism of vacuoles toward survival strategy in plants. Biochim. Biophys. Acta Biomembr. 1862, 183127 (2020).
Payne, R. M. E. et al. An NPF transporter exports a central monoterpene indole alkaloid intermediate from the vacuole. Nat. Plants 3, 16208 (2017).
Rehm, F. B. H. et al. Papain-like cysteine proteases prepare plant cyclic peptide precursors for cyclization. Proc. Natl Acad. Sci. U. S. A. 116, 7831–7836 (2019).
Jackson, M. A. et al. Molecular basis for the production of cyclic peptides by plant asparaginyl endopeptidases. Nat. Commun. 9, 2411 (2018).
Brillouet, J.-M. et al. The tannosome is an organelle forming condensed tannins in the chlorophyllous organs of Tracheophyta. Ann. Bot. 112, 1003–1014 (2013).
Brillouet, J.-M. et al. Phenol homeostasis is ensured in vanilla fruit by storage under solid form in a new chloroplast-derived organelle, the phenyloplast. J. Exp. Bot. 65, 2427–2435 (2014).
Quilichini, T. D., Douglas, C. J. & Samuels, A. L. New views of tapetum ultrastructure and pollen exine development in Arabidopsis thaliana. Ann. Bot. 114, 1189–1201 (2014).
Kim, S. S. & Douglas, C. J. Sporopollenin monomer biosynthesis in Arabidopsis. J. Plant Biol. 56, 1–6 (2013).
Onoyovwe, A. et al. Morphine biosynthesis in opium poppy involves two cell types: sieve elements and laticifers. Plant Cell 25, 4110–4122 (2013).
Li, W., Lybrand, D. B., Zhou, F., Last, R. L. & Pichersky, E. Pyrethrin biosynthesis: the cytochrome P450 oxidoreductase CYP82Q3 converts jasmolone to pyrethrolone. Plant Physiol. 181, 934–944 (2019).
Fu, X. et al. AaPDR3, a PDR transporter 3, is involved in sesquiterpene β-caryophyllene transport in Artemisia annua. Front. Plant Sci. 8, 723 (2017).
Shibata, Y. et al. The full-size ABCG transporters Nb-ABCG1 and Nb-ABCG2 function in pre- and postinvasion defense against Phytophthora infestans in Nicotiana benthamiana. Plant Cell 28, 1163–1181 (2016).
Lefèvre, F. et al. The Nicotiana tabacum ABC transporter NtPDR3 secretes O-methylated coumarins in response to iron deficiency. J. Exp. Bot. 69, 4419–4431 (2018).
Adebesin, F. et al. Emission of volatile organic compounds from petunia flowers is facilitated by an ABC transporter. Science 356, 1386–1388 (2017). This work shows that emission of volatile compounds out of cells relies on active transport and requires the action of an ATP-dependent transporter.
Liao, P. et al. Cuticle thickness affects dynamics of volatile emission from petunia flowers. Nat. Chem. Biol. 17, 138–145 (2021).
Barone, R. P. et al. The production of plant natural products beneficial to humanity by metabolic engineering. Curr. Plant Biol. 24, 100121 (2019).
Achnine, L., Blancaflor, E. B., Rasmussen, S. & Dixon, R. A. Colocalization of l-phenylalanine ammonia-lyase and cinnamate 4-hydroxylase for metabolic channeling in phenylpropanoid biosynthesis. Plant Cell 16, 3098–3109 (2004).
Laursen, T. et al. Characterization of a dynamic metabolon producing the defense compound dhurrin in sorghum. Science 354, 890–893 (2016). By using styrene maleic acid copolymers, this work identifies the metabolon that produces the cyanogenic glucoside dhurrin in Sorghum bicolor.
Mucha, S. et al. The formation of a camalexin biosynthetic metabolon. Plant Cell 31, 2697–2710 (2019).
Gou, M., Ran, X., Martin, D. W. & Liu, C.-J. The scaffold proteins of lignin biosynthetic cytochrome P450 enzymes. Nat. Plants 4, 299–310 (2018).
Zhang, Y. & Fernie, A. R. Metabolons, enzyme–enzyme assemblies that mediate substrate channeling, and their roles in plant metabolism. Plant Commun. 2, 100081 (2020).
Chan, C. Y. et al. Microtubule-directed transport of purine metabolons drives their cytosolic transit to mitochondria. Proc. Natl Acad. Sci. USA 115, 13009–13014 (2018).
Branon, T. C. et al. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 36, 880–887 (2018).
Bar-Even, A. et al. The moderately efficient enzyme: evolutionary and physicochemical trends shaping enzyme parameters. Biochemistry 50, 4402–4410 (2011).
Copley, S. D. An evolutionary biochemist’s perspective on promiscuity. Trends Biochem. Sci. 40, 72–78 (2015).
Akashi, T., Aoki, T. & Ayabe, S.-I. Molecular and biochemical characterization of 2-hydroxyisoflavanone dehydratase. Involvement of carboxylesterase-like proteins in leguminous isoflavone biosynthesis. Plant Physiol. 137, 882–891 (2005).
Kruse, L. H. et al. Ancestral class-promiscuity as a driver of functional diversity in the BAHD acyltransferase family in plants. Preprint at bioRxiv https://doi.org/10.1101/2020.11.18.385815 (2020).
Weng, J.-K. The evolutionary paths towards complexity: a metabolic perspective. N. Phytol. 201, 1141–1149 (2014).
Zhang, J. Evolution by gene duplication: an update. Trends Ecol. Evol. 18, 292–298 (2003).
Storme, N. D., De Storme, N. & Mason, A. Plant speciation through chromosome instability and ploidy change: cellular mechanisms, molecular factors and evolutionary relevance. Curr. Plant Biol. 1, 10–33 (2014).
Shirai, K. & Hanada, K. Contribution of functional divergence through copy number variations to the inter-species and intra-species diversity in specialized metabolites. Front. Plant Sci. 10, 1567 (2019).
Leong, B. J. & Last, R. L. Promiscuity, impersonation and accommodation: evolution of plant specialized metabolism. Curr. Opin. Struct. Biol. 47, 105–112 (2017).
Zhao, Q. et al. The reference genome sequence of Scutellaria baicalensis provides insights into the evolution of wogonin biosynthesis. Mol. Plant 12, 935–950 (2019).
Kaltenbach, M. et al. Evolution of chalcone isomerase from a noncatalytic ancestor. Nat. Chem. Biol. 14, 548–555 (2018).
Cridland, J. M., Majane, A. C., Sheehy, H. K. & Begun, D. J. Polymorphism and divergence of novel gene expression patterns in Drosophila melanogaster. Genetics 216, 79–93 (2020).
Yona, A. H., Alm, E. J. & Gore, J. Random sequences rapidly evolve into de novo promoters. Nat. Commun. 9, 1530 (2018).
Schläpfer, P. et al. Genome-wide prediction of metabolic enzymes, pathways, and gene clusters in plants. Plant Physiol. 173, 2041–2059 (2017).
Banf, M., Zhao, K. & Rhee, S. Y. METACLUSTER—an R package for context-specific expression analysis of metabolic gene clusters. Bioinformatics 35, 3178–3180 (2019).
Nützmann, H.-W. et al. Active and repressed biosynthetic gene clusters have spatially distinct chromosome states. Proc. Natl Acad. Sci. USA 117, 13800–13809 (2020). This work reveals that plant biosynthetic gene clusters reside in highly interactive chromosomal domains that undergo marked changes in local conformation and nuclear positioning as a mechanism for co-regulation of genes in the cluster.
Osbourn, A. Secondary metabolic gene clusters: evolutionary toolkits for chemical innovation. Trends Genet. 26, 449–457 (2010).
Yu, N. et al. Delineation of metabolic gene clusters in plant genomes by chromatin signatures. Nucleic Acids Res. 44, 2255–2265 (2016).
Acknowledgements
This work was supported by grants from the Keck Foundation (J.-K.W.), the Mathers Foundation (J.-K.W.), the Family Larsson-Rosenquist Foundation (J.-K.W.), the National Science Foundation (CHE-1709616 (J.-K.W.) and IOS-1655438 (N.D.)) and Agriculture Hatch (177845 (N.D.)). We thank V.W. Weng for assistance in scientific illustration.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
J.-K.W. is a member of the scientific advisory board and a shareholder of DoubleRainbow Biosciences, Galixir and Inari Agriculture, which develop biotechnologies related to natural products, drug discovery and agriculture. All other authors have declared no competing interests.
Additional information
Peer review information Nature Chemical Biology thanks Matthias Erb and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Weng, JK., Lynch, J.H., Matos, J.O. et al. Adaptive mechanisms of plant specialized metabolism connecting chemistry to function. Nat Chem Biol 17, 1037–1045 (2021). https://doi.org/10.1038/s41589-021-00822-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-021-00822-6
This article is cited by
-
Hordatines, dimerised hydroxycinnamoylagmatine conjugates of barley (Hordeum vulgare L.): an appraisal of the biosynthesis, chemistry, identification and bioactivities
Phytochemistry Reviews (2024)
-
Influences of chemotype and parental genotype on metabolic fingerprints of tansy plants uncovered by predictive metabolomics
Scientific Reports (2023)
-
Emission of floral volatiles is facilitated by cell-wall non-specific lipid transfer proteins
Nature Communications (2023)
-
Chemophenetic and Chemodiversity Approaches: New Insights on Modern Study of Plant Secondary Metabolite Diversity at Different Spatiotemporal and Organizational Scales
Revista Brasileira de Farmacognosia (2022)